Synthesis of NIR-II Absorbing Gelatin Stabilized Gold Nanorods and Its Photothermal Therapy Application against Fibroblast Histiocytoma Cells
Abstract
:1. Introduction
2. Results and Discussion
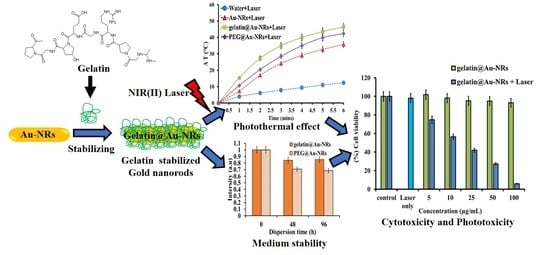
2.1. Synthesis and Characterization of Gelatin@Au-NRs
2.2. Stability of Gelatin@Au-NRs in Biological Media
2.3. Photothermal Profiling Analyses
2.4. Cell Viability and Photothermal Performance Analyses
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Gold Nanorods
3.3. Preparation of Gelatin@Au-NRs
3.4. Preparation of Polyethylene Glycol (mPEG-SH) Capped Au-NRs
3.5. Characterization
3.6. Biological Medium Stability Studies
3.7. Photothermal Profiling Study
3.8. Cell Culture and In Vitro Cytotoxicity Assay
3.9. In Vitro Photothermal Therapy Assay
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amendoeira, A.; García, L.R.; Fernandes, A.R.; Baptista, P.V. Light Irradiation of Gold Nanoparticles Toward Advanced Cancer Therapeutics. Adv. Ther. 2019, 3, 1900153. [Google Scholar] [CrossRef]
- Fanoro, O.; Parani, S.; Maluleke, R.; Lebepe, T.; Varghese, J.; Mavumengwana, V.; Oluwafemi, O. Facile Green, Room-Temperature Synthesis of Gold Nanoparticles Using Combretum erythrophyllum Leaf Extract: Antibacterial and Cell Viability Studies against Normal and Cancerous Cells. Antibiotics 2021, 10, 893. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, A.O.; Lebepe, T.C.; Ncapayi, V.; Tsolekile, N.; Parani, S.; Songca, S.P.; Mori, S.; Kodama, T.; Oluwafemi, O.S. The Therapeutic Effect of Second Near-Infrared Absorbing Gold Nanorods on Metastatic Lymph Nodes via Lymphatic Delivery System. Pharmaceutics 2021, 13, 1359. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, D.; Adewale, O.; Horie, S.; Okajima, J.; Komiya, A.; Oluwafemi, O.; Maruyama, S.; Mori, S.; Kodama, T. Treatment of tumor in lymph nodes using near-infrared laser light-activated thermosensitive liposome-encapsulated doxorubicin and gold nanorods. J. Biophotonics 2017, 10, 1676–1682. [Google Scholar] [CrossRef]
- Park, J.-E.; Kim, M.; Hwang, J.-H.; Nam, J.-M. Golden Opportunities: Plasmonic Gold Nanostructures for Biomedical Applications based on the Second Near-Infrared Window. Small Methods 2017, 1, 1600032. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.-M.; Fang, C.; Jia, H.; Huang, Y.; Cheng, C.H.K.; Ko, C.-H.; Chen, Z.; Wang, J.; Wang, Y.-X.J. Cellular uptake behaviour, photothermal therapy performance, and cytotoxicity of gold nanorods with various coatings. Nanoscale 2014, 6, 11462–11472. [Google Scholar] [CrossRef]
- Lebepe, T.; Parani, S.; Oluwafemi, O. Graphene Oxide-Coated Gold Nanorods: Synthesis and Applications. Nanomaterials 2020, 10, 2149. [Google Scholar] [CrossRef]
- Hlapisi, N.; Motaung, T.E.; Linganiso, L.Z.; Oluwafemi, O.S.; Songca, S.P. Encapsulation of Gold Nanorods with Porphyrins for the Potential Treatment of Cancer and Bacterial Diseases: A Critical Review. Bioinorg. Chem. Appl. 2019, 2019, 1–27. [Google Scholar] [CrossRef]
- Wei, M.-Z.; Deng, T.-S.; Zhang, Q.; Cheng, Z.; Li, S. Seed-Mediated Synthesis of Gold Nanorods at Low Concentrations of CTAB. ACS Omega 2021, 6, 9188–9195. [Google Scholar] [CrossRef]
- Kah, J.C.Y.; Zubieta, A.; Saavedra, R.A.; Hamad-Schifferli, K. Stability of Gold Nanorods Passivated with Amphiphilic Ligands. Langmuir 2012, 28, 8834–8844. [Google Scholar] [CrossRef]
- Horiguchi, Y.; Honda, K.; Kato, Y.; Nakashima, N.; Niidome, Y. Photothermal Reshaping of Gold Nanorods Depends on the Passivating Layers of the Nanorod Surfaces. Langmuir 2008, 24, 12026–12031. [Google Scholar] [CrossRef] [PubMed]
- del Caño, R.; Gisbert-González, J.M.; González-Rodríguez, J.; Sánchez-Obrero, G.; Madueño, R.; Blázquez, M.; Pineda, T. Effective replacement of cetyltrimethylammonium bromide (CTAB) by mercaptoalkanoic acids on gold nanorod (AuNR) surfaces in aqueous solutions. Nanoscale 2020, 12, 658–668. [Google Scholar] [CrossRef]
- Lau, I.P.; Chen, H.; Wang, J.; Ong, H.C.; Leung, K.C.-F.; Ho, H.P.; Kong, S.K. In vitroeffect of CTAB- and PEG-coated gold nanorods on the induction of eryptosis/erythroptosis in human erythrocytes. Nanotoxicology 2011, 6, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Leonov, A.P.; Zheng, J.; Clogston, J.; Stern, S.T.; Patri, A.K.; Wei, A. Detoxification of Gold Nanorods by Treatment with Polystyrenesulfonate. ACS Nano 2008, 2, 2481–2488. [Google Scholar] [CrossRef] [Green Version]
- Terracciano, R.; Zhang, A.; Simeral, M.L.; Demarchi, D.; Hafner, J.H.; Filgueira, C.S. Improvements in Gold Nanorod Biocompatibility with Sodium Dodecyl Sulfate Stabilization. J. Nanotheranostics 2021, 2, 10. [Google Scholar] [CrossRef]
- Mahmoud, N.; Aqabani, H.; Hikmat, S.; Abu-Dahab, R. Colloidal Stability and Cytotoxicity of Polydopamine-Conjugated Gold Nanorods against Prostate Cancer Cell Lines. Molecules 2021, 26, 1299. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Shi, J.; Liu, C.; Li, J.; Cao, S. In-situ self-assembly of sandwich-like Ti3C2 MXene/gold nanorods nanosheets for synergistically enhanced near-infrared responsive drug delivery. Ceram. Int. 2021, 47, 24252–24261. [Google Scholar] [CrossRef]
- Khan, M.S.; Pandey, S.; Bhaisare, M.L.; Gedda, G.; Talib, A.; Wu, H.-F. Graphene oxide@gold nanorods for chemo-photothermal treatment and controlled release of doxorubicin in mice Tumor. Colloids Surf. B Biointerfaces 2017, 160, 543–552. [Google Scholar] [CrossRef]
- Shi, X.; Perry, H.L.; Wilton-Ely, J.D.E.T. Strategies for the functionalisation of gold nanorods to reduce toxicity and aid clinical translation. Nanotheranostics 2021, 5, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Borri, C.; Centi, S.; Ratto, F.; Pini, R. Polylysine as a functional biopolymer to couple gold nanorods to tumor-tropic cells. J. Nanobiotechnol. 2018, 16, 50. [Google Scholar] [CrossRef] [Green Version]
- Nandanan, E.; Jana, N.R.; Ying, J.Y. Functionalization of Gold Nanospheres and Nanorods by Chitosan Oligosaccharide Derivatives. Adv. Mater. 2008, 20, 2068–2073. [Google Scholar] [CrossRef]
- Jakhmola, A.; Vecchione, R.; Onesto, V.; Gentile, F.; Profeta, M.; Battista, E.; Manikas, A.C.; Netti, P.A. A theoretical and experimental study on l-tyrosine and citrate mediated sustainable production of near infrared absorbing twisted gold nanorods. Mater. Sci. Eng. C 2021, 118, 111515. [Google Scholar] [CrossRef] [PubMed]
- Kah, J.C.Y.; Chen, J.; Zubieta, A.; Hamad-Schifferli, K. Exploiting the Protein Corona around Gold Nanorods for Loading and Triggered Release. ACS Nano 2012, 6, 6730–6740. [Google Scholar] [CrossRef] [PubMed]
- Tebbe, M.; Kuttner, C.; Männel, M.; Fery, A.; Chanana, M. Colloidally Stable and Surfactant-Free Protein-Coated Gold Nanorods in Biological Media. ACS Appl. Mater. Interfaces 2015, 7, 5984–5991. [Google Scholar] [CrossRef] [PubMed]
- Grabinski, C.; Schaeublin, N.; Wijaya, A.; D’Couto, H.; Baxamusa, S.H.; Hamad-Schifferli, K.; Hussain, S.M. Effect of Gold Nanorod Surface Chemistry on Cellular Response. ACS Nano 2011, 5, 2870–2879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release 2013, 172, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Du, X.; Wang, T.; Jia, L.; Huang, D.; Chen, W. Synthesis of near-infrared responsive gold nanorod-doped gelatin/hydroxyapatite composite microspheres with controlled photo-thermal property. Ceram. Int. 2018, 44, 900–904. [Google Scholar] [CrossRef]
- Oluwafemi, O.S.; May, B.M.; Parani, S.; Tsolekile, N. Facile, large scale synthesis of water soluble AgInSe2/ZnSe quantum dots and its cell viability assessment on different cell lines. Mater. Sci. Eng. C 2020, 106, 110181. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Kawazoe, N.; Chen, G. Composite scaffolds of gelatin and gold nanoparticles with tunable size and shape for photothermal cancer therapy. J. Mater. Chem. B 2017, 5, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, S.; Zhang, C.; Ikoma, T.; Guo, H.; Zhang, X.; Li, X.; Chen, W. Novel microsphere-packing synthesis, microstructure, formation mechanism and in vitro biocompatibility of porous gelatin/hydroxyapatite microsphere scaffolds. Ceram. Int. 2021, 47, 32187–32194. [Google Scholar] [CrossRef]
- Canpean, V.; Gabudean, A.; Astilean, S. Enhanced thermal stability of gelatin coated gold nanorods in water solution. Colloids Surf. A Physicochem. Eng. Asp. 2013, 433, 9–13. [Google Scholar] [CrossRef]
- Liu, K.; Zheng, Y.; Lu, X.; Thai, T.; Lee, N.A.; Bach, U.; Gooding, J.J. Biocompatible Gold Nanorods: One-Step Surface Functionalization, Highly Colloidal Stability, and Low Cytotoxicity. Langmuir 2015, 31, 4973–4980. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Liu, X.; Deng, G.; Zhou, F.; Zhang, L.; Wang, Q.; Lu, J. Fe3O4@mSiO2-FA-CuS-PEG nanocomposites for magnetic resonance imaging and targeted chemo-photothermal synergistic therapy of cancer cells. Dalton Trans. 2016, 45, 13456–13465. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zheng, C.; Chen, J.; Gao, Y.; Murray, C.B. Using Binary Surfactant Mixtures to Simultaneously Improve the Dimensional Tunability and Monodispersity in the Seeded Growth of Gold Nanorods. Nano Lett. 2013, 13, 765–771. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oladipo, A.; Lebepe, T.C.; Parani, S.; Maluleke, R.; Ncapayi, V.; Mbaz, G.I.M.; Songca, S.P.; Kodama, T.; Oluwafemi, O.S. Synthesis of NIR-II Absorbing Gelatin Stabilized Gold Nanorods and Its Photothermal Therapy Application against Fibroblast Histiocytoma Cells. Pharmaceuticals 2021, 14, 1137. https://doi.org/10.3390/ph14111137
Oladipo A, Lebepe TC, Parani S, Maluleke R, Ncapayi V, Mbaz GIM, Songca SP, Kodama T, Oluwafemi OS. Synthesis of NIR-II Absorbing Gelatin Stabilized Gold Nanorods and Its Photothermal Therapy Application against Fibroblast Histiocytoma Cells. Pharmaceuticals. 2021; 14(11):1137. https://doi.org/10.3390/ph14111137
Chicago/Turabian StyleOladipo, Adewale, Thabang Calvin Lebepe, Sundararajan Parani, Rodney Maluleke, Vuyelwa Ncapayi, Grace It Mwad Mbaz, Sandile Phinda Songca, Tetsuya Kodama, and Oluwatobi Samuel Oluwafemi. 2021. "Synthesis of NIR-II Absorbing Gelatin Stabilized Gold Nanorods and Its Photothermal Therapy Application against Fibroblast Histiocytoma Cells" Pharmaceuticals 14, no. 11: 1137. https://doi.org/10.3390/ph14111137