Defect-Induced Gas-Sensing Properties of a Flexible SnS Sensor under UV Illumination at Room Temperature
Abstract
:1. Introduction
2. Experimental Section
2.1. Sensor Fabrication
2.2. Material Characterization and Gas-Sensing Measurement
2.3. Density Functional Theory Calculations
3. Results and Discussion
3.1. Morphological and Structural Characteristics
3.2. Effects of Sn and S Vacancies on Gas-Sensing Performance
- (i)
- (ii)
- (iii)
- The SnS-80-H250 thin-film sensor exhibited a nil response at all the NO2 concentrations used.
- (iv)
- Film thickness strongly affected the response values and recovery times for both as-deposited and annealed SnS thin-film sensors (300 °C).
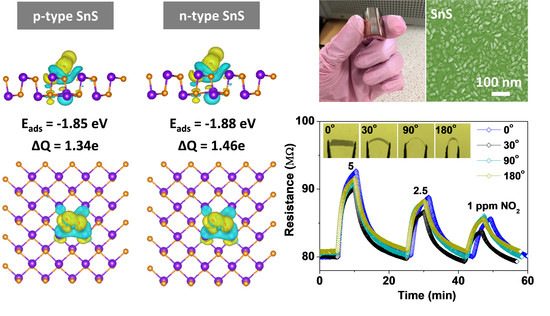
3.3. Gas-Sensing Performance of a Flexible SnS Sensor and the Effect of Humidity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Patil, S.J.; Patil, A.V.; Dighavkar, C.G.; Thakare, K.S.; Borase, R.Y.; Nandre, S.J.; Deshpande, N.G.; Ahire, R.R. Semiconductor metal oxide compounds based gas sensors: A literature review. Front. Mater. Sci. 2015, 9, 14–37. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, S.S.; Kim, H.W. Resistance-based H2S gas sensors using metal oxide nanostructures: A review of recent advances. J. Hazard. Mater. 2018, 357, 314–331. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Li, H.; Yin, Z.; He, Q.; Li, H.; Huang, X.; Lu, G.; Fam, D.W.H.; Tok, A.I.Y.; Zhang, Q.; Zhang, H. Fabrication of single-and multilayer MoS2 film-based field-effect transistors for sensing NO at room temperature. Small 2012, 8, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Li, Z.; Wang, J.; Zhang, J. WS2 nanoflakes based selective ammonia sensors at room temperature. Sens. Actuators B Chem. 2017, 240, 273–277. [Google Scholar] [CrossRef]
- Wang, J.; Lian, G.; Xu, Z.; Fu, C.; Lin, Z.; Li, L.; Wang, Q.; Cui, D.; Wong, C.-P. Growth of large-size SnS thin crystals driven by oriented attachment and applications to gas sensors and photodetectors. ACS Appl. Mater. Interfaces 2016, 8, 9545–9551. [Google Scholar] [CrossRef]
- Ou, J.Z.; Ge, W.; Carey, B.; Daeneke, T.; Rotbart, A.; Shan, W.; Wang, Y.; Fu, Z.; Chrimes, A.F.; Wlodarski, W. Physisorption-based charge transfer in two-dimensional SnS2 for selective and reversible NO2 gas sensing. ACS Nano 2015, 9, 10313–10323. [Google Scholar] [CrossRef]
- Qin, Z.; Xu, K.; Yue, H.; Wang, H.; Zhang, J.; Ouyang, C.; Xie, C.; Zeng, D. Enhanced room-temperature NH3 gas sensing by 2D SnS2 with sulfur vacancies synthesized by chemical exfoliation. Sens. Actuators B Chem. 2018, 262, 771–779. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.; Liang, T.; Sakthivel, T.; Yu, L.; Dai, Z. Activating the Basal Plane of Defective SnS2 Nanosheets for NH3 Gas Sensing. ACS Appl. Nano Mater. 2020, 3, 4642–4653. [Google Scholar] [CrossRef]
- Li, F.; Shi, C. NO-sensing performance of vacancy defective monolayer MoS2 predicted by density function theory. Appl. Surface Sci. 2018, 434, 294–306. [Google Scholar] [CrossRef]
- Kumar, R.; Goel, N.; Kumar, M. UV-activated MoS2 based fast and reversible NO2 sensor at room temperature. ACS Sens. 2017, 2, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Biacchi, A.J.; Le, S.T.; Alberding, B.G.; Hagmann, J.A.; Pookpanratana, S.J.; Heilweil, E.J.; Richter, C.A.; Hight Walker, A.R. Contact and Noncontact Measurement of Electronic Transport in Individual 2D SnS Colloidal Semiconductor Nanocrystals. ACS Nano 2018, 12, 10045–10060. [Google Scholar] [CrossRef] [PubMed]
- Sajeesh, T.H.; Warrier, A.R.; Kartha, C.S.; Vijayakumar, K.P. Optimization of parameters of chemical spray pyrolysis technique to get n and p-type layers of SnS. Thin Solid Films 2010, 518, 4370–4374. [Google Scholar] [CrossRef]
- Afsar, M.; Rafiq, M.; Tok, A. Two-dimensional SnS nanoflakes: Synthesis and application to acetone and alcohol sensors. RSC Adv. 2017, 7, 21556. [Google Scholar] [CrossRef] [Green Version]
- Ristov, M.; Sinadinovski, G.; Grozdanov, I.; Mitreski, M. Chemical deposition of tin (II) sulphide thin films. Thin Solid Films 1989, 173, 53–58. [Google Scholar] [CrossRef]
- Chinh, N.D.; Hien, T.T.; Do Van, L.; Hieu, N.M.; Quang, N.D.; Lee, S.-M.; Kim, C.; Kim, D. Adsorption/desorption kinetics of nitric oxide on zinc oxide nano film sensor enhanced by light irradiation and gold-nanoparticles decoration. Sens. Actuators B Chem. 2019, 281, 262–272. [Google Scholar] [CrossRef]
- Chinh, N.D.; Kim, C.; Kim, D. UV-light-activated H2S gas sensing by a TiO2 nanoparticulate thin film at room temperature. J. Alloy. Compd. 2019, 778, 247–255. [Google Scholar] [CrossRef]
- Hung, N.M.; Hieu, N.M.; Chinh, N.D.; Hien, T.T.; Quang, N.D.; Majumder, S.; Choi, G.; Kim, C.; Kim, D. Rb2CO3-decorated In2O3 nanoparticles for the room-temperature detection of sub-ppm level NO2. Sens. Actuators B Chem. 2020, 313, 128001. [Google Scholar] [CrossRef]
- Xia, J.; Li, X.-Z.; Huang, X.; Mao, N.; Zhu, D.-D.; Wang, L.; Xu, H.; Meng, X.-M. Physical vapor deposition synthesis of two-dimensional orthorhombic SnS flakes with strong angle/temperature-dependent Raman responses. Nanoscale 2016, 8, 2063–2070. [Google Scholar] [CrossRef]
- Catherall, A.L.; Harris, S.; Hill, M.S.; Johnson, A.L.; Mahon, M.F. Deposition of SnS Thin Films from Sn(II) Thioamidate Precursors. Crystal Growth Design 2017, 17, 5544–5551. [Google Scholar] [CrossRef] [Green Version]
- Parkin, I.P.; Price, L.S.; Hibbert, T.G.; Molloy, K.C. The first single source deposition of tin sulfide coatings on glass: Aerosol-assisted chemical vapour deposition using [Sn(SCHCHS)]. J. Mater. Chem. 2001, 11, 1486–1490. [Google Scholar] [CrossRef]
- Ramasamy, K.; Kuznetsov, V.L.; Gopal, K.; Malik, M.A.; Raftery, J.; Edwards, P.P.; O’Brien, P. Organotin dithiocarbamates: Single-source precursors for tin sulfide thin films by aerosol-assisted chemical vapor deposition (AACVD). Chem. Mater. 2013, 25, 266–276. [Google Scholar] [CrossRef]
- Shafiei, M.; Bradford, J.; Khan, H.; Piloto, C.; Wlodarski, W.; Li, Y.; Motta, N. Low-operating temperature NO2 gas sensors based on hybrid two-dimensional SnS2-reduced graphene oxide. Appl. Surface Sci. 2018, 462, 330–336. [Google Scholar] [CrossRef]
- Bharatula, L.D.; Erande, M.B.; Mulla, I.S.; Rout, C.S.; Late, D.J. SnS2 nanoflakes for efficient humidity and alcohol sensing at room temperature. RSC Adv. 2016, 6, 105421–105427. [Google Scholar] [CrossRef]
- Arepalli, V.K.; Nguyen, T.D.; Kim, J. Influence of Ag thickness on the structural, optical, and electrical properties of the SnS/Ag/SnS trilayer films for solar cell application. Curr. Appl. Phys. 2020, 20, 438–444. [Google Scholar] [CrossRef]
- Gedi, S.; Reddy, V.R.M.; Kang, J.-y.; Jeon, C.-W. Impact of high temperature and short period annealing on SnS films deposited by E-beam evaporation. Appl. Surf. Sci. 2017, 402, 463–468. [Google Scholar] [CrossRef]
- Sharma, D.; Kamboj, N.; Agarwal, K.; Mehta, B.R. Structural, optical and photoelectrochemical properties of phase pure SnS and SnS2 thin films prepared by vacuum evaporation method. J. Alloys Compd. 2020, 822, 153653. [Google Scholar] [CrossRef]
- Reddy, T.S.; Kumar, M.C.S. Co-evaporated SnS thin films for visible light photodetector applications. RSC Adv. 2016, 6, 95680–95692. [Google Scholar] [CrossRef]
- Fu, X.; Ilanchezhiyan, P.; Mohan Kumar, G.; Cho, H.D.; Zhang, L.; Chan, A.S.; Lee, D.J.; Panin, G.N.; Kang, T.W. Tunable UV-visible absorption of SnS2 layered quantum dots produced by liquid phase exfoliation. Nanoscale 2017, 9, 1820–1826. [Google Scholar] [CrossRef]
- Burton, L.A.; Whittles, T.J.; Hesp, D.; Linhart, W.M.; Skelton, J.M.; Hou, B.; Webster, R.F.; O’Dowd, G.; Reece, C.; Cherns, D.; et al. Electronic and optical properties of single crystal SnS2: An earth-abundant disulfide photocatalyst. J. Mater. Chem. A 2016, 4, 1312–1318. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Song, G.; Li, W.; Peng, Y.; Jiang, L.; Xue, Y.; Liu, Q.; Chen, Z.; Hu, J. Phase-controlled synthesis and photocatalytic properties of SnS, SnS2 and SnS/SnS2 heterostructure nanocrystals. Mater. Res. Bull. 2013, 48, 2325–2332. [Google Scholar] [CrossRef]
- Mittal, K.L. Polyimides and Other High Temperature Polymers: Synthesis, Characterization and Applications; CRC Press: Boca Raton, FL, USA, 2005; Volume 3. [Google Scholar]
- Moulder, J.F. Handbook of X-ray photoelectron spectroscopy; Physical Electron: Eden Prairie, MN, USA, 1995; pp. 230–232. ISBN 0-9648124-1-X. [Google Scholar]
- Cheng, S.; Conibeer, G. Physical properties of very thin SnS films deposited by thermal evaporation. Thin Solid Films 2011, 520, 837–841. [Google Scholar] [CrossRef]
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill: New York, NY, USA, 2003; Volume 529. [Google Scholar]
- Ricciardella, F.; Vollebregt, S.; Polichetti, T.; Miscuglio, M.; Alfano, B.; Miglietta, M.L.; Massera, E.; Di Francia, G.; Sarro, P.M. Effects of graphene defects on gas sensing properties towards NO2 detection. Nanoscale 2017, 9, 6085–6093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Liu, H.; Chu, Y.; Cui, Y.; Hayasaka, T.; Dasaka, V.; Nguyen, L.; Lin, L. Defect-Induced Gas Adsorption on Graphene Transistors. Adv. Mater. Interfaces 2018, 5, 1701640. [Google Scholar] [CrossRef]
- Kim, J.; Choi, S.-W.; Lee, J.-H.; Chung, Y.; Byun, Y.T. Gas sensing properties of defect-induced single-walled carbon nanotubes. Sens. Actuators B Chem. 2016, 228, 688–692. [Google Scholar] [CrossRef]
- Navale, S.T.; Chougule, M.A.; Patil, V.B.; Mane, A.T. Highly sensitive, reproducible, selective and stable CSA-polypyrrole NO2 sensor. Synth. Metals 2014, 189, 111–118. [Google Scholar] [CrossRef]
- Chougule, M.A.; Sen, S.; Patil, V.B. Polypyrrole–ZnO hybrid sensor: Effect of camphor sulfonic acid doping on physical and gas sensing properties. Synth. Metals 2012, 162, 1598–1603. [Google Scholar] [CrossRef]
- Jiang, X.; Tai, H.; Ye, Z.; Yuan, Z.; Liu, C.; Su, Y.; Jiang, Y. Novel p-n heterojunction-type rGO/CeO2 bilayer membrane for room-temperature nitrogen dioxide detection. Mater. Lett. 2017, 186, 49–52. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Lee, D.-S.; Choi, H.K.; Lee, D.H.; Kim, J.-E.; Lee, J.Y.; Lee, W.J.; Kim, S.O.; Choi, S.-Y. Flexible room-temperature NO2 gas sensors based on carbon nanotubes/reduced graphene hybrid films. Appl. Phys. Lett. 2010, 96, 213105. [Google Scholar] [CrossRef] [Green Version]
- Pyeon, J.J.; Baek, I.-H.; Song, Y.G.; Kim, G.S.; Cho, A.-J.; Lee, G.-Y.; Han, J.H.; Chung, T.-M.; Hwang, C.S.; Kang, C.-Y. Highly sensitive flexible NO2 sensor composed of vertically aligned 2D SnS2 operating at room temperature. J. Mater. Chem. C 2020. [Google Scholar] [CrossRef]
- Yan, X.; Wu, Y.; Li, R.; Shi, C.; Moro, R.; Ma, Y.; Ma, L. High-Performance UV-Assisted NO2 Sensor Based on Chemical Vapor Deposition Graphene at Room Temperature. ACS Omega 2019, 4, 14179–14187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, N.M.; Chinh, N.D.; Nguyen, T.D.; Kim, E.T.; Choi, G.; Kim, C.; Kim, D. Carbon nanotube-metal oxide nanocomposite gas sensing mechanism assessed via NO2 adsorption on n-WO3/p-MWCNT nanocomposites. Ceram. Int. 2020. [Google Scholar] [CrossRef]









| Samples | Sn (at%) | S (at%) | O (at%) | C (at%) | Sn/S |
|---|---|---|---|---|---|
| As-deposited SnS-80 | 12.29 | 23.82 | 21.70 | 42.19 | 0.52 |
| SnS-80-H250 | 12.28 | 11.47 | 32.39 | 43.86 | 1.07 |
| SnS-80-H300 | 18.01 | 8.33 | 49.75 | 23.91 | 2.16 |
| Samples | Conductivity (Ω−1cm−1) | Carrier Concentration (cm−3) | Mobility (cm2/V.s) | Carrier Type |
|---|---|---|---|---|
| As-deposited SnS-80 | 3.65 × 10−5 | 3.83 × 1012 | 59.5 | p-type |
| SnS-80-H250 | - | - | - | - |
| SnS-80-H300 | 4.3 × 10−4 | 3.7 × 1013 | 72.5 | n-type |
| Monolayer Structures | Eads (eV) | ΔQ (e) |
|---|---|---|
| SnS | −0.68 | 1.03 |
| SnS-Sn | −1.85 | 1.34 |
| SnS-S | −1.88 | 1.46 |
| Materials | [NO2]/ppm | T/°C | Response (%) | tres/trec | Reference |
|---|---|---|---|---|---|
| a CSA-PPy | 100 | RT | 36 | 250 s/40 min | [39] |
| a PPy-ZnO | 10 | RT | 15 | 10 min/3 h | [40] |
| a rGO/CeO2 | 10 | RT | 20 | 10 min/10 min | [41] |
| b CNT/reduced graphene | 5 | RT | 13 | -/over 60 min | [42] |
| b SnS2 flakes/PI | 1 | RT | 50 | 8 min/30 min | [43] |
| b SnS2 flakes/Al2O3/PI | 5 | RT | 309 | 8 min/over 30 min | [43] |
| b Graphene | 100 | RT | 26 | 5 min/over 15 min | [44] |
| SnS thin-film | 5 | RT | 18 | 5 min/12 min | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manh Hung, N.; Nguyen, C.V.; Arepalli, V.K.; Kim, J.; Duc Chinh, N.; Nguyen, T.D.; Seo, D.-B.; Kim, E.-T.; Kim, C.; Kim, D. Defect-Induced Gas-Sensing Properties of a Flexible SnS Sensor under UV Illumination at Room Temperature. Sensors 2020, 20, 5701. https://doi.org/10.3390/s20195701
Manh Hung N, Nguyen CV, Arepalli VK, Kim J, Duc Chinh N, Nguyen TD, Seo D-B, Kim E-T, Kim C, Kim D. Defect-Induced Gas-Sensing Properties of a Flexible SnS Sensor under UV Illumination at Room Temperature. Sensors. 2020; 20(19):5701. https://doi.org/10.3390/s20195701
Chicago/Turabian StyleManh Hung, Nguyen, Chuong V. Nguyen, Vinaya Kumar Arepalli, Jeha Kim, Nguyen Duc Chinh, Tien Dai Nguyen, Dong-Bum Seo, Eui-Tae Kim, Chunjoong Kim, and Dojin Kim. 2020. "Defect-Induced Gas-Sensing Properties of a Flexible SnS Sensor under UV Illumination at Room Temperature" Sensors 20, no. 19: 5701. https://doi.org/10.3390/s20195701