A Gold Nanoclusters Film Supported on Polydopamine for Fluorescent Sensing of Free Bilirubin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instrumentations
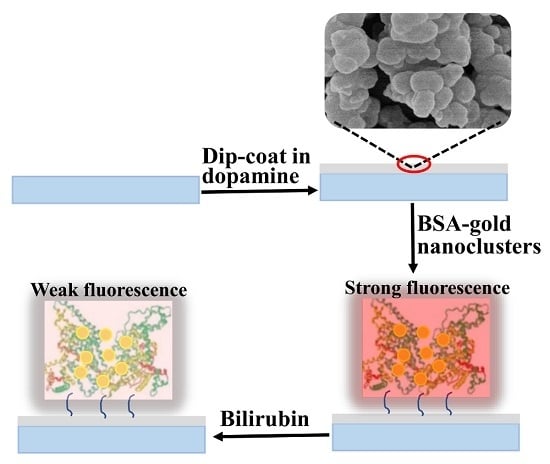
2.2. Preparation of the Gold Nanoclusters Film by Polydopamine Adhesion
2.3. Fluorescence Measurements
3. Results and Discussion
3.1. Characterization of PDA/AuNCs Film
3.2. PDA/AuNCs Film for Fluorescent Sensing of fBR
3.3. Determination of fBR in Human Blood Serum
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fevery, J. Bilirubin in clinical practice: A review. Liver Int. 2008, 28, 592–605. [Google Scholar] [CrossRef]
- Westwood, A. The analysis of bilirubin in serum. Ann. Clin. Biochem. 1991, 28, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T. Mechanisms of bilirubin toxicity: Clinical implications. Clin. Perinatol. 2002, 29, 765–778. [Google Scholar] [CrossRef]
- Chen, S.; Lu, W.; Yueh, M.; Rettenmeier, E.; Liu, M.; Auwerx, J.; Yu, R.; Evans, R.; Wang, K.; Karind, M. Intestinal NCoR1, a regulator of epithelial cell maturation, controls neonatal hyperbilirubinemia. Proc. Natl. Acad. Sci. USA 2017, 114, E1432–E1440. [Google Scholar] [PubMed] [Green Version]
- Shapiro, S. Bilirubin toxicity in the developing nervous system. Pediatr. Neurol. 2003, 29, 410–421. [Google Scholar] [CrossRef]
- Ahlfors, C.; Wennberg, R.; Ostrow, J.; Tiribelli, C. Unbound (free) bilirubin: Improving the paradigm for evaluating neonatal jaundice. Clin. Chem. 2009, 55, 1288–1299. [Google Scholar] [PubMed]
- Ameri, M.; Schnaars, H.; Sibley, J.; Honor, D. Comparison of the vanadate oxidase method with the diazo method for serum bilirubin determination in dog, monkey, and rat. J. Vet. Diagn. Investig. 2011, 123, 120–123. [Google Scholar]
- Batra, B.; Lata, S.; Rana, J.; Pundir, C.S. Construction of an amperometric bilirubin biosensor based on covalent immobilization of bilirubin oxidase onto zirconia coated silica nanoparticles/chitosan hybrid film. Biosens. Bioelectron. 2013, 44, 64–69. [Google Scholar] [PubMed]
- Noh, H.; Won, M.; Shim, Y. Selective nonenzymatic bilirubin detection in blood samples using a nafion/mn–cu sensor. Biosens. Bioelectron. 2014, 61, 554–561. [Google Scholar] [CrossRef]
- Martelanc, M.; Žiberna, L.; Passamonti, S.; Franko, M. Direct determination of fBR in serum at sub-nanomolar levels. Anal. Chim. Acta 2014, 809, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Moein, M.M.; Jabbar, D.; Colmsjö, A.; Abdelrehim, M. A needle extraction utilizing a molecularly imprinted-sol-gel xerogel for on-line microextraction of the lung cancer biomarker bilirubin from plasma and urine samples. J. Chromatogr. A 2014, 1366, 15–23. [Google Scholar] [CrossRef]
- Sun, H.; Nie, Z.; Fung, Y.S. Determination of fBR and its binding capacity by HSA using a microfluidic chip-capillary electrophoresis device with a multi-segment circular-ferrofluid-driven micromixing injection. Electrophoresis 2010, 31, 3061–3069. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Jin, R.; Kong, D.S.; Zhao, X.; Liu, F.M.; Yan, X.; Lin, Y.H.; Lu, G.Y. Switchable fluorescence immunoassay using gold nanoclusters anchored cobalt oxyhydroxide composite for sensitive detection of imidacloprid. Sens. Actuators B Chem. 2019, 283, 207–214. [Google Scholar] [CrossRef]
- Xiao, W.; Han, G.; Chen, Z. Ratiometric fluorescent sensing of copper ion based on a pyrene schiff base langmuir-blodgett film. Sens. Lett. 2015, 13, 501–505. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, Z. Fluorescent iron (III) determination based on salicylaldehyde functionalized bimodal mesoporous silica. J. Nanosci. Nanotechnol. 2016, 16, 12666–12670. [Google Scholar] [CrossRef]
- Kohashi, K.; Date, Y.; Morita, M.; Tsuruta, Y. Fluorescence reaction of bilirubin with zinc ion in dimethyl sulfoxide and its application to assay of total bilirubin in serum. Anal. Chim. Acta 1998, 365, 177–182. [Google Scholar] [CrossRef]
- Wabaidur, S.M.; Eldesoky, G.E.; Alothman, Z.A. The fluorescence quenching of Ru(bipy)32+: An application for the determination of bilirubin in biological samples. Luminescence 2018, 33, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Kamruzzaman, M.; Alam, A.M.; Hak Lee, S.; Ho Kim, Y.; Kim, G.M.; Hyub Oh, S. Spectrofluorimetric quantification of bilirubin using yttrium–norfloxacin complex as a fluorescence probe in serum samples. J. Lumin. 2012, 132, 3053–3057. [Google Scholar] [CrossRef]
- Jayasree, M.; Aparna, R.S.; Anjana, R.R.; Devi, J.S.A.; John, N.; Abha, K.; Manikandan, A.; George, S. Fluorescence turn on detection of bilirubin using Fe (III) modulated BSA stabilized copper nanocluster; a mechanistic perception. Anal. Chim. Acta 2018, 1031, 152–160. [Google Scholar] [CrossRef]
- Zhang, M.M.; Xu, L.Y.; Ma, Q.B.; Yu, H.; Fang, H.F.; Lin, Z.X.; Zhang, Q.L.; Chen, Z. A pH-Controlled Kit for Total and Direct Bilirubin Built on Mimetic Peroxidase CoFe2O4-DOPA-Catalyzed Fluorescence Enhancement. ACS Appl. Mater. Interfaces 2018, 10, 42155–42164. [Google Scholar] [CrossRef]
- Senthilkumar, T.; Asha, S.K. Selective and sensitive sensing of fBR in human serum using water-soluble polyfluorene as fluorescent probe. Macromolecules 2015, 48, 3449–3461. [Google Scholar] [CrossRef]
- Du, Y.; Li, X.; Lv, X.; Jia, Q. Highly sensitive and selective sensing of fBR using metal-organic frameworks-based energy transfer process. ACS Appl. Mater. Interfaces 2017, 9, 30925–30932. [Google Scholar] [CrossRef]
- Ellairaja, S.; Shenbagavalli, K.; Ponmariappan, S.; Vasantha, V.S. A green and facile approach for synthesizing imine to develop optical biosensor for wide range detection of bilirubin in human biofluids. Biosens. Bioelectron. 2017, 91, 82–88. [Google Scholar] [CrossRef]
- Chen, L.Y.; Wang, C.W.; Yuan, Z.; Chang, H.T. Fluorescent gold nanoclusters: Recent advances in sensing and imaging. Anal. Chem. 2015, 87, 216–229. [Google Scholar] [CrossRef]
- Zhuo, C.X.; Wang, L.H.; Feng, J.J.; Zhang, Y.D. Label-free fluorescent detection of trypsin activity based on DNA-stabilized silver nanocluster-peptide conjugates. Sensors 2016, 16, 1477–1486. [Google Scholar] [CrossRef]
- Li, H.; Jin, R.; Kong, D.; Zhao, X.; Liu, F.; Yan, X.; Lin, Y.; Lu, G. A Dual-Emission Fluorescent Nanocomplex of Gold-Cluster-Decorated Silica Particles for Live Cell Imaging of Highly Reactive Oxygen Species. J. Am. Chem. Soc. 2013, 135, 11595–11602. [Google Scholar]
- Abbas, M.A.; Kim, T.Y.; Lee, S.U.; Kang, Y.S.; Bang, J.H. Exploring interfacial events in gold-nanocluster-sensitized solar cells: Insights into the effects of the cluster size and electrolyte on solar cell performance. J. Am. Chem. Soc. 2016, 138, 390–401. [Google Scholar] [CrossRef]
- Cho, S.; Shin, H.Y.; Kim, M.I. Nanohybrids consisting of magnetic nanoparticles and gold nanoclusters as effective peroxidase mimics and their application for colorimetric detection of glucose. Biointerphases 2017, 12, 01A401. [Google Scholar] [CrossRef]
- Lin, Z.; Luo, F.; Dong, T.; Zheng, L.; Wang, Y.; Chi, Y.; Chen, G. Recyclable fluorescent gold nanocluster membrane for visual sensing of copper(ii) ion in aqueous solution. Analyst 2012, 137, 2394–2399. [Google Scholar] [CrossRef]
- Wu, R.H.; Yau, S.H.; Iii, T.G. Linear and nonlinear optical properties of monolayer-protected gold nanocluster films. ACS Nano 2016, 10, 562–572. [Google Scholar] [CrossRef]
- Pu, Z.; Yi, W.; Yin, Y. Facile fabrication of a gold nanocluster-based membrane for the detection of hydrogen peroxide. Sensors 2016, 16, 1124–1134. [Google Scholar]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef]
- Yan, L.; Cai, Y.; Zheng, B.; Yuan, H.; Guo, Y.; Xiao, D.; Choi, M.M.F. Microwave-assisted synthesis of BSA-stabilized and HSA-protected gold nanoclusters with red emission. J. Mater. Chem. 2011, 22, 1000–1005. [Google Scholar] [CrossRef]
- Santhosh, M.; Chinnadayyala, S.R.; Kakoti, A.; Goswami, P. Selective and sensitive detection of fBR in blood serum using human serum albumin stabilized gold nanoclusters as fluorometric and colorimetric probe. Biosens. Bioelectron. 2014, 59, 370–376. [Google Scholar] [CrossRef]
- Hayashi, Y.; Kawada, Y.; Ichimura, K. Dicyanoanthracene as a fluorescence probe for studies on silica surfaces. Langmuir 1995, 11, 2077–2082. [Google Scholar] [CrossRef]
- Fang, Y.; Ning, G.; Hu, D.; Lu, J. Synthesis and solvent-sensitive fluorescence properties of a novel surface-functionalized chitosan film: Potential materials for reversible information storage. J. Photochem. Photobiol. A Chem. 2000, 135, 141–145. [Google Scholar] [CrossRef]






| Sample | Determined (μM) | Spiked (μM) | Found a (μM) | RSD b (%) | Recovery c (%) |
|---|---|---|---|---|---|
| Sample 1 | 5.73 ± 0.34 | 5.00 | 10.96 ± 0.28 | 2.6 | 104.6 |
| 20.00 | 25.65 ± 1.10 | 4.3 | 99.6 | ||
| Sample 2 | 7.85 ± 0.45 | 5.00 | 12.92 ± 0.31 | 2.4 | 101.4 |
| 20.00 | 27.88 ± 1.31 | 4.7 | 100.2 | ||
| Sample 3 | 15.87 ± 1.08 | 5.00 | 20.78 ± 0.66 | 3.2 | 98.2 |
| 20.00 | 36.02 ± 1.30 | 3.6 | 100.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Xiao, W.; Huang, R.; Shi, Y.; Fang, C.; Chen, Z. A Gold Nanoclusters Film Supported on Polydopamine for Fluorescent Sensing of Free Bilirubin. Sensors 2019, 19, 1726. https://doi.org/10.3390/s19071726
Li Z, Xiao W, Huang R, Shi Y, Fang C, Chen Z. A Gold Nanoclusters Film Supported on Polydopamine for Fluorescent Sensing of Free Bilirubin. Sensors. 2019; 19(7):1726. https://doi.org/10.3390/s19071726
Chicago/Turabian StyleLi, Zhou, Wenxiang Xiao, Rongen Huang, Yajing Shi, Cheng Fang, and Zhencheng Chen. 2019. "A Gold Nanoclusters Film Supported on Polydopamine for Fluorescent Sensing of Free Bilirubin" Sensors 19, no. 7: 1726. https://doi.org/10.3390/s19071726