Highly Fluorescent Green Carbon Dots as a Fluorescent Probe for Detecting Mineral Water pH
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterizations
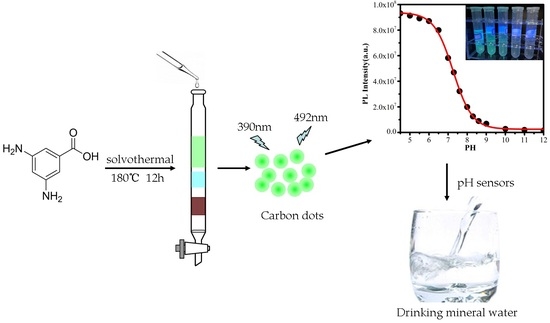
2.2. Preparation of CDs and pH Buffer Solutions
2.3. QY Measurements
2.4. Method for Detecting the pH Value of Mineral Water
3. Results
3.1. Characterizations of the CDs
3.2. The Optical Properties of the CDs
3.3. Fluorescent Response of the CDs to pH
3.4. Resistance to Temperature and Ion Interference and Luminescence Stability
3.5. pH Detection in Mineral Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.; Wang, H.; Luo, P.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D.; et al. Carbon dots for multiphoton bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Yu, S.B.; Wei, J.S.; Xiong, H.M. Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism. ACS Nano 2015, 10, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, Green and blue luminescence by carbon dots: Full-color emission tuning and multicolor cellular imaging. Angew. Chem. Int. Ed. 2015, 127, 5450–5453. [Google Scholar] [CrossRef]
- Pan, L.; Sun, S.; Zhang, A.; Jiang, K.; Zhang, L.; Dong, C.; Huang, Q.; Wu, A.; Lin, H. Truly fluorescent excitation-dependent carbon dots and their applications in multicolor cellular imaging and multidimensional sensing. Adv. Mater. 2015, 27, 7782–7787. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, M.; Xiao, Y.; Dong, H.; Zhang, H.; Zhuang, J.; Hu, H.; Lei, B.; Liu, Y. A self quenching resistant carbon dot powder with tunable solid state fluorescence and construction of dual fluorescence morphologies for white light emission. Adv. Mater. 2016, 28, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Ma, J.; Shan, X.; Feng, H.; Shao, L.; Chen, J. Highly luminescent N-doped carbon quantum dots as an effective multifunctional fluorescence sensing platform. Chem. Eur. J. 2014, 20, 2254–2263. [Google Scholar] [CrossRef]
- Tan, H.C.; Zhao, W.H.; Qi, Q.; Zhang, R.; Zuo, Y.Y.; Yang, L.J. Green synthesis of nitrogen-doped fluorescent carbon quantum dots for selective detection of iron. Fullerenes Nanotubes Carbon Nanostruct. 2017, 25, 417–422. [Google Scholar] [CrossRef]
- Luo, L.; Wang, P.; Wang, Y.; Wang, F. pH assisted selective detection of Hg(II) and Ag(I) based on nitrogen-rich carbon dots. Sens. Actuators Chem. 2018, 273, 1640–1647. [Google Scholar] [CrossRef]
- Ding, Y.Y.; Gong, X.J.; Liu, Y.; Lu, W.J.; Gao, Y.F.; Xian, M.; Shuang, S.M.; Dong, C. Facile preparation of bright orange fluorescent carbon dots and the constructed biosensing platform for the detection of pH in living cells. Talanta 2018, 189, 8–15. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lei, Y. Fluorescent carbon dots and their sensing applications. Trends Anal. Chem. 2017, 89, 163–180. [Google Scholar] [CrossRef]
- Sharma, S.; Umar, A.; Sood, S.; Mehta, S.K.; Kansal, S.K. Photoluminescent C-dots: An overview on the recent development in the synthesis, physiochemical properties and potential applications. J. Alloys Compd. 2018, 748, 818–853. [Google Scholar] [CrossRef]
- Shangguan, J.; He, D.; He, X.; Wang, K.; Xu, F.; Liu, J.; Huang, J. Label-Free Carbon-Dots-Based Ratiometric Fluorescence pH nanoprobes for intracellular pH sensing. Anal. Chem. 2016, 88, 7837–7843. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.; Zhang, L.; Shi, B.; Chen, D.; Zhao, L.; Zhao, S. Fluorescent carbon dots with tunable emission by dopamine for sensing of intracellular pH, elementary arithmetic operations and a living cell imaging based INHIBIT logic gate. J. Mater. Chem. B 2017, 5, 5265–5271. [Google Scholar] [CrossRef]
- Jiao, Y.; Gong, X.; Han, H.; Gao, Y.; Lu, W.; Liu, Y.; Dong, C. Facile synthesis of orange fluorescence carbon dots with excitation independent emission for pH sensing and cellular imaging. Anal. Chim. Acta 2018, 1042, 125–132. [Google Scholar] [CrossRef]
- Nie, H.; Li, M.; Li, Q.; Liang, S.; Tan, Y.; Sheng, L.; Shi, W.; Zhang, S.X.A. Carbon dots with continuously tunable full-color emission and their application in ratiometric pH sensing. Chem. Mater. 2014, 26, 3104–3112. [Google Scholar] [CrossRef]
- Barati, A.; Shamsipur, M.; Abdollahi, H. Carbon dots with strong excitation-dependent fluorescence changes towards pH. Application as nanosensors for a broad range of pH. Anal. Chim. Acta 2016, 931, 25–33. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Z.; Zhang, C. Polyethyleneimine-functionalized fluorescent carbon dots: Water stability, pH sensing, and cellular imaging. Chem. Nano. Mat. 2015, 1, 122–127. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Wang, C.; Tong, D.; Wu, Q.; Jiang, K.; Yang, M. Red emitting and highly stable carbon dots with dual response to pH values and ferric ions. Microchim. Acta 2018, 185, 1–8. [Google Scholar] [CrossRef]
- Zhang, M.; Su, R.; Zhong, J.; Fei, L.; Cai, W.; Guan, Q.; Xu, Q. Red/orange dual-emissive carbon dots for pH sensing and cell imaging. J. Nano Res. 2019, 12, 815–821. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Z.; Cheng, H.; Lin, H.; Humphrey, M.G.; Zhang, C. A hydrothermal route to water-stable luminescent carbon dots as nanosensors for pH and temperature. Carbon 2015, 82, 87–95. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.; Li, W.; Han, Y.; Liu, Y.; Li, Z.; Zhang, B.; Pan, D. Rationally designed an efficient dual-mode colorimetric/fluorescence sensor based on carbon dots for detection of pH and Cu2+ ions. ACS Sustain. Chem. Eng. 2018, 1–18. [Google Scholar] [CrossRef]
- Song, Z.; Quan, F.; Xu, Y.; Liu, M.; Cui, L.; Liu, J. Multifunctional N, S co-doped carbon quantum dots with pH-and thermo-dependent switchable fluorescent properties and highly selective detection of glutathione. Carbon 2016, 104, 169–178. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Aseer, K.R.; Perumal, S.; Karthik, N.; Lee, Y.R. Highly fluorescent nitrogen-doped carbon dots derived from Phyllanthus acidus utilized as a fluorescent probe for label-free selective detection of Fe3+ ions, live cell imaging and fluorescent ink. Biosens. Bioelectron. 2018, 99, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, W. Nitrogen-doped carbon quantum dots: Facile synthesis and application as a “turn-off” fluorescent probe for detection of Hg2+ ions. Biosens. Bioelectron. 2014, 55, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Zhou, F.; Gu, J.; Shu, C.; Xi, K.; Jia, X. Nitrogen-doped carbon dots as a new substrate for sensitive glucose determination. Sensors 2016, 16, 630. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, J.; Zhai, Y.; Wang, H.; Bai, X.; Dong, B.; Song, H. A novel mechanism for red emission carbon dots: Hydrogen bond dominated molecular states emission. Nanoscale 2017, 9, 13042–13051. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. J. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Wang, H.; Sun, C.; Chen, X.; Zhang, Y.; Colvin, V.L.; Rice, Q.; Seo, J.; Feng, S.; Wang, S.; Yu, W.W. Excitation wavelength independent visible color emission of carbon dots. Nanoscale 2017, 9, 1909–1915. [Google Scholar] [CrossRef]
- Jiang, K.; Sun, S.; Zhang, L.; Wang, Y.; Cai, C.; Lin, H. Bright-yellow-emissive N-doped carbon dots: Preparation, cellular imaging, and bifunctional sensing. ACS Appl. Mater. Interfaces 2015, 7, 23231–23238. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Gong, X.; Yang, Z.; Zhang, Y.; Hu, Q.; Shuang, S.; Dong, C.; Choi, M.M.F. High-quality water-soluble luminescent carbon dots for multicolor patterning, sensors, and bioimaging. RSC Adv. 2015, 5, 16972–16979. [Google Scholar] [CrossRef]
- Lu, X.; Liu, C.; Wang, Z.; Yang, J.; Xu, M.; Dong, J.; Wang, P.; Gu, J.; Cao, F. Nitrogen-doped carbon nanoparticles derived from silkworm excrement as on–off–on fluorescent sensors to detect Fe(III) and biothiols. Nanomaterials 2018, 8, 443. [Google Scholar] [CrossRef] [PubMed]




| Mineral Water Brand | pH a | pH b |
|---|---|---|
| Ganten | 5.93 | 5.9 |
| NongFu (suitable for infants) | 7.13 | 7.3 |
| NongFu | 7.34 | 7.4 |
| Alps | 6.80 | 6.6 |
| LIFELONG | 7.74 | 7.7 |
| Evergrande Spring (low sodium) | 7.21 | 7.2 |
| Perrier (carbonated water) | 5.32 | 6.2 |
| Wahaha (soda water) | 8.09 | 7.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Chen, G.; Li, L.; Wu, Y. Highly Fluorescent Green Carbon Dots as a Fluorescent Probe for Detecting Mineral Water pH. Sensors 2019, 19, 3801. https://doi.org/10.3390/s19173801
Wang T, Chen G, Li L, Wu Y. Highly Fluorescent Green Carbon Dots as a Fluorescent Probe for Detecting Mineral Water pH. Sensors. 2019; 19(17):3801. https://doi.org/10.3390/s19173801
Chicago/Turabian StyleWang, Tingyu, Guoqing Chen, Lei Li, and Yamin Wu. 2019. "Highly Fluorescent Green Carbon Dots as a Fluorescent Probe for Detecting Mineral Water pH" Sensors 19, no. 17: 3801. https://doi.org/10.3390/s19173801