Beyond the Hydro-Regime: Differential Regulation of Plant Functional Groups in Seasonal Ponds
Abstract
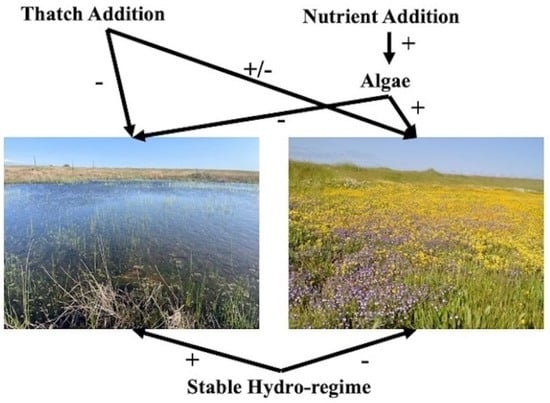
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Plant Sampling
2.3. Statistical Analysis
3. Results
3.1. Macroalgae
3.2. Plants
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McIntyre, S.; Díaz, S.; Lavorel, S.; Cramer, W. Plant functional types and disturbance dynamics–Introduction. J. Veg. Sci. 1999, 10, 603–608. [Google Scholar] [CrossRef]
- Collinge, S.K.; Ray, C. Transient patterns in the assembly of vernal pool plant communities. Ecology 2009, 90, 3313–3323. [Google Scholar] [CrossRef] [PubMed]
- Van der Knaap, Y.A.M.; Aerts, R.; Van Bodegom, P.M. Is the differential response of riparian plant performance to extreme drought and inundation events related to differences in intraspecific trait variation? Funct. Plant Biol. 2014, 41, 609–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraft, N.J.B.; Crutsinger, G.M.; Forrestel, E.J.; Emery, N.C. Functional trait differences and the outcome of community assembly: An experimental test with vernal Pool annual plants. Oikos 2014, 23, 1391–1399. [Google Scholar] [CrossRef]
- Komatsu, K.J.; Avolio, M.L.; Lemoine, N.P.; Isbell, F.; Grman, E.; Houseman, G.R.; Koerner, S.E.; Johnson, D.S.; Wilcox, K.R.; Alatalo, J.M.; et al. Global change effects on plant communities are magnified by time and the number of global change factors imposed. Proc. Natl. Acad. Sci. USA 2019, 116, 17867–17873. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, J.; Roscher, C. Differential effects of functional traits on aboveground biomass in semi-natural grasslands. Oikos 2009, 118, 1659–1668. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of Maintenance of Species Diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef] [Green Version]
- Bliss, S.A.; Zedler, P.H. The Germination Process in Vernal Pools: Sensitivity to Environmental Conditions and Effects on Community Structure. Oecologia 1998, 113, 67–73. [Google Scholar] [CrossRef]
- Tiner, R.W. Using Plants as Indicators of Wetland. Proc. Acad. Nat. Sci. Phila. 1993, 144, 240–253. [Google Scholar]
- Euliss, N.H.; LaBaugh, J.W.; Fredrickson, L.H.; Mushet, D.M.; Laubhan, M.K.; Swanson, G.A.; Winter, T.C.; Rosenberry, D.O.; Nelson, R.D. The Wetland Continuum: A Conceptual Framework for Interpreting Biological Studies. Wetlands 2004, 24, 448–458. [Google Scholar] [CrossRef] [Green Version]
- Van der Valk, A. The prairie potholes of North America. In The World’s Largest Wetlands: Ecology and Conservation; Fraser, L., Keddy, P., Eds.; Cambridge University Pres: Cambridge, UK, 2005; pp. 393–423. [Google Scholar]
- Toner, M.; Keddy, P. River hydrology and riparian wetlands: A predictive model for ecological assembly. Ecol. Appl. 1997, 7, 236–246. [Google Scholar] [CrossRef]
- Kirkman, L.K.; Goebel, P.C.; West, L.; Drew, M.B.; Palik, B.J. Depressional wetland vegetation types: A question of plant community development. Wetlands 2000, 20, 373–385. [Google Scholar] [CrossRef]
- Warwick, N.W.M.; Brock, M.A. Plant reproduction in temporary wetlands: The effects of seasonal timing, depth, and duration of flooding. Aquat. Bot. 2003, 77, 153–167. [Google Scholar] [CrossRef]
- Capon, S.J.; Brock, M.A. Flooding, soil seed bank dynamics and vegetation resilience of a hydrologically variable desert floodplain. Freshw. Biol. 2006, 51, 206–223. [Google Scholar] [CrossRef]
- Miao, S.; Zou, C.B.; Breshears, D.D. Vegetation responses to extreme hydrological events: Sequence matters. Am. Nat. 2009, 173, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Gerhardt, F.; Collinge, S.K. Abiotic constraints eclipse biotic resistance in determining invasibility along experimental vernal pool gradients. Ecol. Appl. A Publ. Ecol. Soc. Am. 2009, 17, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Bauder, E.T. Inundation effects on small-scale plant distributions in San Diego, California vernal pools. Aquat. Ecol. 2000, 34, 43–61. [Google Scholar] [CrossRef]
- Platenkamp, G.A.J. Patterns of vernal pool biodiversity at Beale Air Force Base. In Ecology, Conservation and Management of Vernal Pool Ecosystems; Witham, C.W., Bauder, E.T., Belk, D., Ferren, W.R., Ornduff, R., Eds.; California Native Plant Society: Sacramento, CA, USA, 1998; pp. 151–160. [Google Scholar]
- Capon, S. Plant community responses to wetting and drying in a large arid floodplain. River Res. Appl. 2003, 19, 509–520. [Google Scholar] [CrossRef]
- Kneitel, J.M. Inundation timing, more than duration, affects the community structure of California vernal pool mesocosms. Hydrobiologia 2014, 732, 71–83. [Google Scholar] [CrossRef]
- Gosejohan, M.C.; Weisberg, P.J.; Merriam, K.E. Hydrologic influences on plant community structure in vernal pools of northeastern California. Wetlands 2017, 37, 257–268. [Google Scholar] [CrossRef]
- Pätzig, M.; Kalettka, T.; Glemnitz, M.; Berger, G. What governs macrophyte species richness in kettle hole types? A case study from Northeast Germany. Limnol.-Ecol. Manag. Inland Waters 2012, 42, 340–354. [Google Scholar] [CrossRef]
- Kneitel, J.M.; Lessin, C.L. Ecosystem-phase interactions: Aquatic eutrophication decreases terrestrial plant diversity in California vernal pools. Oecologia 2010, 163, 461–469. [Google Scholar] [CrossRef]
- Amatangelo, K.L.; Dukes, J.S.; Field, C.B. Responses of a California annual grassland to litter manipulation. J. Veg. Sci. 2008, 19, 605–612. [Google Scholar] [CrossRef]
- Loydi, A.; Eckstein, L.; Otte, A.; Donath, T. Effects of litter on seedling establishment in natural and semi-natural grasslands: A meta-analysis. J. Ecol. 2013, 101, 454–464. [Google Scholar] [CrossRef]
- Faist, A.M.; Beals, S.C. Invasive plant feedbacks promote alternative states in California vernal pools. Restor. Ecol. 2018, 26, 255–263. [Google Scholar] [CrossRef]
- Churchill, A.C.; Faist, A.M. Consequences of above-ground invasion by non-native plants into restored vernal pools do not prompt same changes in below-ground processes. AoB Plants 2021, 13, plab042. [Google Scholar] [CrossRef] [PubMed]
- Croel, R.C.; Kneitel, J.M. Cattle waste reduces plant diversity in vernal pool mesocosms. Aquat. Bot. 2011, 95, 140–145. [Google Scholar] [CrossRef]
- Kido, R.R.; Kneitel, J.M. Eutrophication effects differ among functional groups in vernal pool invertebrate communities. Hydrobiologia 2021, 848, 1659–1673. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Avolio, M.L.; Koerner, S.E.; La Pierre, K.; Wilcox, K.; Wilson, G.; Smith, M.; Collins, S. Changes in plant community composition, not diversity, during a decade of nitrogen and phosphorus additions drive above-ground productivity in a tallgrass prairie. J. Ecol. 2014, 102, 1649–1660. [Google Scholar] [CrossRef] [Green Version]
- Krause-Jensen, D.; McGlathery, K.; Rysgaard, S.; Christensen, P.B. Production within dense mats of the filamentous macroalga Chaetomorpha linum in relation to light and nutrient availability. Mar. Ecol. Prog. Ser. 1996, 134, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Faist, A.M.; Ferrenberg, S.; Collinge, S.K. Banking on the past: Seed banks as a reservoir for rare and native species in restored vernal pools. AoB Plants 2013, 5, plt043. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, B.G.; Goldman, D.H.; Keil, D.J.; Patterson, R.; Rosatti, T.J. (Eds.) The Jepson Manual: Vascular Plants of California; University of California Press: Berkeley, CA, USA, 2012. [Google Scholar]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Wetlands and Water Synthesis; World Resources Institute: Washington, DC, USA, 2005. [Google Scholar]
- Bartolome, J.W.; Jackson, R.D.; Betts, A.D.K.; Connor, J.M.; Nader, G.A.; Tate, K.W. Effects of residual dry matter on net primary production and plant functional groups in Californian annual grasslands. Grass Forage Sci. 2007, 62, 445–452. [Google Scholar] [CrossRef]
- Smith, M.J.; Kay, W.R.; Edward, D.H.D.; Papas, P.J.; Richardson, K.S.J.; Simpson, J.C.; Halse, S.A. AusRivAS: Using macroinvertebrates to assess ecological condition of rivers in Western Australia. Freshw. Biol. 1999, 41, 269–282. [Google Scholar] [CrossRef]
- Boven, L.; Stoks, R.; Forró, L.; Brendonck, L. Seasonal dynamics in water quality and vegetation cover in temporary pools with variable hydroperiods in Kiskunság (Hungary). Wetlands 2008, 28, 401–410. [Google Scholar] [CrossRef]
- Zhang, P.; Ayumi, K.; Van Leeuwen, C.H.A.; Velthuis, M.; Van Donk, E.; Xu, J.; Bakker, E.S. Interactive effects of rising temperature and nutrient enrichment on aquatic plant growth, stoichiometry, and palatability. Front. Plant Sci. 2020, 11, 58. [Google Scholar] [CrossRef] [Green Version]
- Wijewardene, L.; Wu, N.; Fohrer, N.; Riis, T. Epiphytic biofilms in freshwater and interactions with macrophytes: Current understanding and future directions. Aquat. Bot. 2022, 176, 103–467. [Google Scholar] [CrossRef]
- Hautier, Y.; Niklaus, P.A.; Hector, A. Competition for light causes plant biodiversity loss after eutrophication. Science 2009, 324, 636–638. [Google Scholar] [CrossRef] [Green Version]
- Power, M.E. Benthic turfs vs floating mats of algae in river food webs. Oikos 1990, 58, 67–79. [Google Scholar] [CrossRef]
- Bai, Y.; Han, X.; Wu, J.; Chen, Z.; Li, L. Ecosystem stability and compensatory effects in the inner Mongolia grassland. Nature 2004, 431, 181–184. [Google Scholar] [CrossRef]
- Scheffer, M.; Rinaldi, S.; Gragnani, A.; Mur, L.R.; Van Nes, E.H. On the dominance of filamentous cyanobacteria in shallow, turbid lakes. Ecology 1997, 78, 272–282. [Google Scholar] [CrossRef]
- Wu, X.; Mitsch, W.J. Spatial and temporal patterns of algae in newly constructed freshwater wetlands. Wetlands 1998, 18, 9–20. [Google Scholar] [CrossRef]
- Marazzi, L.; Gaiser, E.E.; Jones, V.J.; Tobias, F.A.; Mackay, A.W. Algal richness and life-history strategies are influenced by hydrology and phosphorus in two major subtropical wetlands. Freshw. Biol. 2017, 62, 274–290. [Google Scholar] [CrossRef]
- Grime, J.P. The role of seed dormancy in vegetation dynamics. Ann. Appl. Biol. 1981, 98, 555–558. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Carta, A.; Bedini, G.; Mueller, J.V.; Probert, R.J. Comparative seed dormancy and germination of eight annual species of ephemeral wetland vegetation in a Mediterranean climate. Plant Ecol. 2013, 214, 339–349. [Google Scholar] [CrossRef]
- Xiong, S.; Nilsson, C. The effects of plant litter on vegetation: A meta-analysis. J. Ecol. 1999, 87, 984–994. [Google Scholar] [CrossRef]
- Collinge, S.K.; Ray, C.; Marty, J.T. A long-term comparison of hydrology and plant community composition in constructed versus naturally occurring vernal pools. Restor. Ecol. 2013, 21, 704–712. [Google Scholar] [CrossRef]
- Heady, H.F. Vegetational changes in the California annual type. Ecology 1958, 39, 402–416. [Google Scholar] [CrossRef]
- Pitt, M.D.; Heady, H.F. Responses of annual vegetation to temperature and rainfall patterns in northern California. Ecology 1978, 59, 336–350. [Google Scholar] [CrossRef]
- Facelli, J.M.; Pickett, S.T. Plant litter: Its dynamics and effects on plant community structure. Bot. Rev. 1991, 57, 1–32. [Google Scholar] [CrossRef]
- Molinari, N.A.; D’Antonio, C.M. Where have all the wildflowers gone? The role of exotic grass thatch. Biol. Invasions 2020, 22, 957–968. [Google Scholar] [CrossRef]
- Hassan, N.; Sher, K.; Rab, A.; Abdullah, I.; Zeb, U.; Naeem, I.; Khan, A. Effects and mechanism of plant litter on grassland ecosystem: A review. Acta Ecol. Sin. 2021, 41, 341–345. [Google Scholar] [CrossRef]
- Weltzin, J.F.; Keller, J.K.; Bridgham, S.D.; Pastor, J.; Allen, P.B.; Chen, J. Litter controls plant community composition in a northern fen. Oikos 2005, 110, 537–546. [Google Scholar] [CrossRef]
- Craft, C.; Kull, K.; Graham, S. Ecological indicators of nutrient enrichment, freshwater wetlands, Midwestern United States. Ecol. Indic. 2007, 7, 733–750. [Google Scholar] [CrossRef]
- Veen, G.F.; Fry, E.L.; Ten Hooven, F.C.; Kardol, P.; Morriën, E.; De Long, J.R. The role of plant litter in driving plant-soil feedbacks. Front. Environ. Sci. 2019, 7, 168. [Google Scholar] [CrossRef]
- Marty, J. Fire effects on plant biodiversity across multiple sites in California vernal pool grasslands. Ecol. Restor. 2015, 33, 266–273. [Google Scholar] [CrossRef]
- Marty, J.T. Loss of biodiversity and hydrologic function in seasonal wetlands persists over 10 years of livestock grazing removal. Restor. Ecol. 2015, 23, 548–554. [Google Scholar] [CrossRef]
- Bailey, D.W.; Mosley, J.C.; Estell, R.E.; Cibils, A.F.; Horney, M.; Hendrickson, J.R.; Burritt, E.A. Synthesis paper: Targeted livestock grazing: Prescription for healthy rangelands. Rangel. Ecol. Manag. 2019, 72, 865–877. [Google Scholar] [CrossRef]
- Michaels, J.; Batzer, E.; Harrison, S.; Eviner, V.T. Grazing affects vegetation diversity and heterogeneity in California vernal pools. Ecology 2021, 102, e03295. [Google Scholar] [CrossRef]
- Wei, L.; Tan, R.; Yu-ming, Y.; Wang, J. Plant diversity as a good indicator of vegetation stability in a typical plateau wetland. J. Mt. Sci. 2014, 11, 464–474. [Google Scholar]
- Spivak, A.C.; Vanni, M.J.; Mette, E.M. Moving on up: Can results from simple aquatic mesocosm experiments be applied across broad spatial scales? Freshw. Biol. 2011, 56, 279–291. [Google Scholar] [CrossRef]
- Batzer, D.P. The seemingly intractable ecological responses of invertebrates in North American wetlands: A review. Wetlands 2013, 13, 1–15. [Google Scholar] [CrossRef]
- Sardans, J.; Penuelas, J. The role of plants in the effects of global change on nutrient availability and stoichiometry in the plant soil system. Plant Physiol. 2012, 160, 1741–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Species | Habitat Distribution | Hydro-Phase Affiliation |
|---|---|---|
| Cladophora sp. 1 | Widespread | Aquatic |
| Nitella sp. 1 | Widespread | Aquatic |
| Eleocharis macrostachya | Endemic | Aquatic |
| Callitriche marginata | Endemic | Aquatic |
| Ranunculus aquatilis | Widespread | Aquatic |
| Marsilea vestita | Widespread | Aquatic |
| Plagiobothrys stipitatus | Endemic | Terrestrial |
| Downwingia bicornuta | Endemic | Terrestrial |
| Gratiola ebracteata | Endemic | Terrestrial |
| Navarretia leucocephala | Endemic | Terrestrial |
| Psilocarphus brevissimus | Endemic | Terrestrial |
| Dependent Variable | Independent Variable | Wald Chi-Square | df | p |
|---|---|---|---|---|
| Algae | ||||
| Year | 54.313 | 1 | <0.001 | |
| Hydroperiod | 52.147 | 1 | <0.001 | |
| Thatch | 3.170 | 2 | 0.205 | |
| Nutrient | 5.925 | 1 | 0.015 | |
| Year * Hydroperiod | 40.840 | 1 | <0.001 | |
| Year * Thatch | 12.052 | 2 | 0.002 | |
| Year * Nutrient | 2.023 | 1 | 0.155 | |
| Hydroperiod * Thatch | 0.158 | 2 | 0.924 | |
| Hydroperiod * Nutrient | 5.163 | 1 | 0.023 | |
| Thatch * Nutrient | 1.985 | 2 | 0.371 | |
| Year * Hydroperiod * Thatch | 3.295 | 2 | 0.193 | |
| Year * Hydroperiod * Nutrient | 9.122 | 1 | 0.003 | |
| Year * Thatch * Nutrient | 6.624 | 2 | 0.036 | |
| Hydroperiod * Thatch * Nutrient | 3.089 | 2 | 0.213 | |
| Year * Hydroperiod * Thatch * Nutrient | 8.485 | 1 | 0.004 | |
| Species richness | ||||
| Year | 17.856 | 1 | <0.001 | |
| Hydroperiod | 36.815 | 1 | <0.001 | |
| Thatch | 39.877 | 2 | <0.001 | |
| Nutrient | 13.598 | 1 | <0.001 | |
| Year * Hydroperiod | 0.802 | 1 | 0.370 | |
| Year * Thatch | 2.906 | 2 | 0.234 | |
| Year * Nutrient | 0.359 | 1 | 0.549 | |
| Hydroperiod * Thatch | 3.998 | 2 | 0.135 | |
| Hydroperiod * Nutrient | 0.941 | 1 | 0.332 | |
| Thatch * Nutrient | 7.599 | 2 | 0.022 | |
| Year * Hydroperiod * Thatch | 1.999 | 2 | 0.368 | |
| Year * Hydroperiod * Nutrient | 3.287 | 1 | 0.070 | |
| Year * Thatch * Nutrient | 1.205 | 2 | 0.548 | |
| Hydroperiod * Thatch * Nutrient | 5.252 | 2 | 0.072 | |
| Year * Hydroperiod * Thatch * Nutrient | 1.230 | 2 | 0.541 | |
| Total percent cover | ||||
| Year | 78.223 | 1 | <0.001 | |
| Hydroperiod | 8.152 | 1 | 0.004 | |
| Thatch | 5.888 | 2 | 0.050 | |
| Nutrient | 0.010 | 1 | 0.922 | |
| Year * Hydroperiod | 1.893 | 1 | 0.169 | |
| Year * Thatch | 0.729 | 2 | 0.694 | |
| Year * Nutrient | 0.021 | 1 | 0.886 | |
| Hydroperiod * Thatch | 6.671 | 2 | 0.036 | |
| Hydroperiod * Nutrient | 2.629 | 1 | 0.105 | |
| Thatch * Nutrient | 5.555 | 2 | 0.062 | |
| Year * Hydroperiod * Thatch | 1.825 | 2 | 0.401 | |
| Year * Hydroperiod * Nutrient | 2.014 | 1 | 0.156 | |
| Year * Thatch * Nutrient | 3.353 | 2 | 0.187 | |
| Hydroperiod * Thatch * Nutrient | 1.800 | 2 | 0.407 | |
| Year * Hydroperiod * Thatch * Nutrient | 2.375 | 2 | 0.305 | |
| Aquatic plants | ||||
| Year | 1.300 | 1 | 0.254 | |
| Hydroperiod | 5.561 | 1 | 0.018 | |
| Thatch | 7.684 | 2 | 0.021 | |
| Nutrient | 0.412 | 1 | 0.521 | |
| Year * Hydroperiod | 0.192 | 1 | 0.661 | |
| Year * Thatch | 6.134 | 2 | 0.047 | |
| Year * Nutrient | 0.753 | 1 | 0.386 | |
| Hydroperiod * Thatch | 4.378 | 2 | 0.112 | |
| Hydroperiod * Nutrient | 2.008 | 1 | 0.156 | |
| Thatch * Nutrient | 1.583 | 2 | 0.453 | |
| Year * Hydroperiod * Thatch | 2.324 | 2 | 0.313 | |
| Year * Hydroperiod * Nutrient | 0.228 | 1 | 0.633 | |
| Year * Thatch * Nutrient | 1.004 | 2 | 0.605 | |
| Hydroperiod * Thatch * Nutrient | 1.916 | 2 | 0.384 | |
| Year * Hydroperiod * Thatch * Nutrient | 1.931 | 2 | 0.381 | |
| Terrestrial plants | ||||
| Year | 163.145 | 1 | <0.001 | |
| Hydroperiod | 0.446 | 1 | 0.504 | |
| Thatch | 10.387 | 2 | 0.006 | |
| Nutrient | 0.087 | 1 | 0.768 | |
| Year * Hydroperiod | 0.016 | 1 | 0.898 | |
| Year * Thatch | 8.300 | 2 | 0.016 | |
| Year * Nutrient | 0.087 | 1 | 0.768 | |
| Hydroperiod * Thatch | 4.703 | 2 | 0.095 | |
| Hydroperiod * Nutrient | 0.042 | 1 | 0.837 | |
| Thatch * Nutrient | 0.887 | 2 | 0.642 | |
| Year * Hydroperiod * Thatch | 6.902 | 2 | 0.032 | |
| Year * Hydroperiod * Nutrient | 0.111 | 1 | 0.739 | |
| Year * Thatch * Nutrient | 1.930 | 2 | 0.381 | |
| Hydroperiod * Thatch * Nutrient | 1.715 | 2 | 0.424 | |
| Year * Hydroperiod * Thatch * Nutrient | 1.035 | 2 | 0.596 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rios, J.; Dibbell, M.; Flores, E.; Kneitel, J.M. Beyond the Hydro-Regime: Differential Regulation of Plant Functional Groups in Seasonal Ponds. Diversity 2023, 15, 832. https://doi.org/10.3390/d15070832
Rios J, Dibbell M, Flores E, Kneitel JM. Beyond the Hydro-Regime: Differential Regulation of Plant Functional Groups in Seasonal Ponds. Diversity. 2023; 15(7):832. https://doi.org/10.3390/d15070832
Chicago/Turabian StyleRios, Jasmine, Melanie Dibbell, Emely Flores, and Jamie M. Kneitel. 2023. "Beyond the Hydro-Regime: Differential Regulation of Plant Functional Groups in Seasonal Ponds" Diversity 15, no. 7: 832. https://doi.org/10.3390/d15070832