Macro-moth (Lepidoptera) Diversity of a Newly Shaped Ecological Corridor and the Surrounding Forest Area in the Western Italian Alps
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
Topographic and Cover Variable Estimation
2.3. Data Analysis
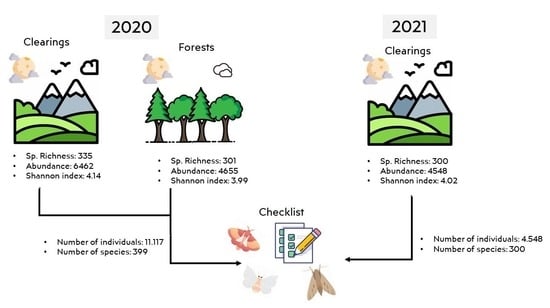
3. Results
4. Discussion
- -
- Eupithecia gueneata (Millière, 1862) and Eupithecia spissilineata (Metzner, 1846) are new for the moth fauna of Piedmont. E. gueneata (Millière, 1862) is a Western-Palaearctic species, inhabiting warm and dry habitats where its caterpillar feeds on Pimpinella saxifrage L. [48], known in Southern and Eastern Europe, with only isolated data reported for Italy. In Piedmont, recent unpublished observations report its occurrence not far from the study area, in the Alpi Cozie Natural Park (Massimo Rosso pers. comm.). Conversely, E. spissilineata is an Eastern-Mediterranean species, inhabiting rocky habitats with an unknown larval biology [47], with the westernmost populations being known in Southeastern France. Since, in Italy, this species has only been observed in Latium, Abruzzo, and doubtfully in Tuscany [32], the data reported in this paper significantly extends its Italian range northwards.
- -
- -
- Synopsia sociaria (Hübner, 1799), Colostygia pectinataria (Knoch, 1781), Epirrita christyi (Allen, 1906), Hydriomena impluviata (Denis and Schiffermüller, 1775), Perizoma blandiata (Denis and Schiffermüller, 1775), Charissa (Costignophos) pullata (Denis and Schiffermüller, 1775), Scopula (Scopula) submutata (Treitschke, 1828), Cucullia (Shargacucullia) lychnitis (Rambur, 1833), Cucullia (Shargacucullia) prenanthis (Boisduval, 1840), Apamea aquila (Donzel, 1837), Hoplodrina alsinides (Constantini, 1922), Mythimna (Anapoma) riparia (Rambur, 1829), Noctua interposita (Hübner, 1790), Grammodes stolida (Fabricius, 1775), and Nola confusalis (Herrich-Schäffer, 1847) are new to the moth fauna of Susa Valley, according to [38];
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrew, C.; Heegaard, E.; Kirk, P.M.; Bässler, C.; Heilmann-Clausen, J.; Krisai-Greilhuber, I.; Kuyper, T.W.; Senn-Irlet, B.; Büntgen, U.; Diez, J.; et al. Big data integration: Pan-European fungal species observations’ assembly for addressing contemporary questions in ecology and global change biology. Fungal Biol. Rev. 2017, 31, 88–98. [Google Scholar] [CrossRef]
- Soroye, P.; Ahmed, N.; Kerr, J.T. Opportunistic citizen science data transform understanding of species distributions, phenology, and diversity gradients for global change research. Glob. Chang. Biol. 2018, 24, 5281–5291. [Google Scholar] [CrossRef] [PubMed]
- McGeoch, M.A. The selection, testing and application of terrestrial insects as bioindicators. Biol. Rev. 1998, 73, 181–201. [Google Scholar] [CrossRef]
- Gerlach, J.; Samways, M.; Pryke, J. Terrestrial invertebrates as bioindicators: An overview of available taxonomic groups. J. Insect Conserv. 2013, 17, 831–850. [Google Scholar] [CrossRef]
- Dar, A.A.; Jamal, K. Moths as ecological indicators: A review. Munis Entomol. Zool. J. 2021, 16, 830–836. [Google Scholar]
- Hill, G.M.; Kawahara, A.Y.; Daniels, J.C.; Bateman, C.C.; Scheffers, B.R. Climate change effects on animal ecology: Butterflies and moths as a case study. Biol. Rev. 2021, 96, 2113–2126. [Google Scholar] [CrossRef]
- da Rocha, J.R.M.; De Almeida, J.R.; Lins, G.A.; Durval, A. Insects as indicators of environmental changing and pollution: A review of appropriate species and their monitoring. Holos Environ. 2010, 10, 250–262. [Google Scholar] [CrossRef]
- Siegloch, A.E.; Schmitt, R.; Spies, M.; Petrucio, M.; Hernández, M.I.M. Effects of small changes in riparian forest complexity on aquatic insect bioindicators in Brazilian subtropical streams. Mar. Freshw. Res. 2016, 68, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Enkhtur, K.; Pfeiffer, M.; Lkhagva, A.; Boldgiv, B. Response of moths (Lepidoptera: Heterocera) to livestock grazing in Mongolian rangelands. Ecol. Indic. 2017, 72, 667–674. [Google Scholar] [CrossRef]
- Greco, S.; Infusino, M.; Ienco, A.; Scalercio, S. How different management regimes of chestnut forests affect diversity and abundance of moth communities? Ann. Silvic. Res. 2018, 42, 59–67. [Google Scholar]
- Uhl, B.; Wölfling, M.; Bässler, C. Mediterranean moth diversity is sensitive to increasing temperatures and drought under climate change. Sci. Rep. 2022, 12, 1–10. [Google Scholar]
- Muscolo, A.; Bagnato, S.; Sidari, M.; Mercurio, R. A review of the roles of forest canopy gaps. J. For. Res. 2014, 25, 725–736. [Google Scholar] [CrossRef]
- Merckx, T.; Feber, R.E.; Hoare, D.J.; Parsons, M.S.; Kelly, C.J.; Bourn, N.A.; Macdonald, D.W. Conserving threatened Lepidoptera: Towards an effective woodland management policy in landscapes under intense human land-use. Biol. Conserv. 2012, 149, 32–39. [Google Scholar] [CrossRef]
- Kozel, P.; Sebek, P.; Platek, M.; Benes, J.; Zapletal, M.; Dvorsky, M.; Lanta, V.; Dolezal, J.; Bace, R.; Zbuzek, B.; et al. Connectivity and succession of open structures as a key to sustaining light-demanding biodiversity in deciduous forests. J. Appl. Ecol. 2021, 58, 2951–2961. [Google Scholar] [CrossRef]
- Tasser, E.; Walde, J.; Tappeiner, U.; Teutsch, A.; Noggler, W. Land-Use Changes and Natural Reforestation in the Eastern Central Alps. Agric. Ecosyst. Environ. 2007, 118, 115–129. [Google Scholar] [CrossRef]
- Orlandi, S.; Probo, M.; Sitzia, T.; Trentanovi, G.; Garbarino, M.; Lombardi, G.; Lonati, M. Environmental and land use determinants of grassland patch diversity in the western and eastern Alps under agropastoral abandonment. Biodivers. Conserv. 2016, 25, 275–293. [Google Scholar] [CrossRef]
- Riva, F.; Acorn, J.H.; Nielsen, S.E. Narrow anthropogenic corridors direct the movement of a generalist boreal butterfly. Biol. Lett. 2018, 14, 20170770. [Google Scholar] [CrossRef] [Green Version]
- Öckinger, E.; Smith, H.G. Do corridors promote dispersal in grassland butterflies and other insects? Landsc. Ecol. 2008, 23, 27–40. [Google Scholar] [CrossRef]
- Berg, Å.; Bergman, K.O.; Wissman, J.; Żmihorski, M.; Öckinger, E. Power-line corridors as source habitat for butterflies in forest landscapes. Biol. Conserv. 2016, 201, 320–326. [Google Scholar] [CrossRef]
- Parmesan, C.; Ryrholm, N.; Stefanescu, C.; Hill, J.K.; Thomas, C.D.; Descimon, H.; Huntleyk, B.; Kaila, L.; Kullberg, J.; Tammaru, T.; et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 1999, 399, 579–583. [Google Scholar] [CrossRef]
- Lenoir, J.; Gegout, J.C.; Marquet, P.A.; de Ruffray, P.; Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- D’Amen, M.; Pradervand, J.N.; Guisan, A. Predicting richness and composition in mountain insect communities at high resolution: A new test of the SESAM framework. Glob. Ecol. Biogeogr. 2015, 24, 1443–1453. [Google Scholar] [CrossRef] [Green Version]
- Didham, R.K.; Basset, Y.; Collins, C.M.; Leather, S.R.; Littlewood, N.A.; Menz, M.H.; Müller, J.; Packer, L.; Saunders, M.E.; Schönrogge, K.; et al. Interpreting insect declines: Seven challenges and a way forward. Insect Conserv. Divers. 2020, 13, 103–114. [Google Scholar] [CrossRef]
- Ehrlén, J.; Morris, W.F. Predicting changes in the distribution and abundance of species under environmental change. Ecol. Lett. 2015, 18, 303–314. [Google Scholar] [CrossRef] [Green Version]
- Hudson, A. Biosystematics in the genus Euxoa (Lepidoptera: Noctuidae). Can. Entomol. 1973, 105, 1199–1209. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PloS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotes, B.; Ruano, F.; García, P.A.; Pascual, F.; Campos, M. Coccinellid morphospecies as an alternative method for differentiating management regimes in olive orchards. Ecol. Indic. 2009, 9, 548–555. [Google Scholar] [CrossRef]
- Derraik, J.G.; Early, J.W.; Closs, G.P.; Dickinson, K.J. Morphospecies and taxonomic species comparison for Hymenoptera. J. Insect Sci. 2010, 10, 108. [Google Scholar] [CrossRef] [Green Version]
- Mattila, N.; Kaitala, V.; Komonen, A.; Kotiaho, J.S.; Päivinen, J. Ecological determinants of distribution decline and risk of extinction in moths. Conserv. Biol. 2006, 20, 1161–1168. [Google Scholar] [CrossRef]
- Hahn, M.; Brühl, C.A. The secret pollinators: An overview of moth pollination with a focus on Europe and North America. Arthropod-Plant Interact. 2016, 10, 21–28. [Google Scholar] [CrossRef]
- Lees, D.; Zilli, A. Moths. A Complete Guide to Biology and Behavior; Natural History Museum: London, UK, 2019; pp. 5–8. [Google Scholar]
- Parenzan, P.; Porcelli, F. I macrolepidotteri italiani. Fauna Lepidopterorum Italiae (Macrolepidoptera)—Addenda et corrigenda. I. Entomologica 2008, 40, 153–221. [Google Scholar]
- Parile, E.; Piccini, I.; Bonelli, S. A demographic and ecological study of an Italian population of Polyommatus ripartii: The ESU Polyommatus exuberans. J. Insect Conserv. 2021, 25, 783–796. [Google Scholar] [CrossRef]
- Anselmo, L. New distributional data of the protected butterfly Papilio alexanor Esper, 1800 in north-western Italy and some ecological observations (Lepidoptera papilionidae). Biodivers. J. 2021, 12, 21–26. [Google Scholar] [CrossRef]
- Piccini, I.; Luca, C.; Di Pietro, V.; Bonelli, S.; Biscaccianti, A.B. A revision of distribution, ecology and conservation issues of the threatened comb-claw beetle Gerandryus aetnensis (Coleoptera: Tenebrionidae, Alleculinae). Fragm. Entomol. 2021, 53, 13–20. [Google Scholar]
- Anselmo, L.; Rizzioli, B. The small range and the great threat: Extinction risk assessment of the narrow endemism Carabus cychroides under climate change. J. Insect Conserv. 2022, 26, 17–27. [Google Scholar] [CrossRef]
- Piccini, I.; Di Pietro, V.; Bonelli, S. Zerynthia polyxena locally monophagous on Aristolochia pallida in the Susa Valley. Environ. Entomol. 2021, 50, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, F.; Bertaccini, E. I Macrolepidotteri della Valle di Susa. Italia Nord-Occidentale (Alpi Cozie-Graie); Museo Regionale di Scienze Naturali: Turin, Italy, 2004; p. 389. [Google Scholar]
- Heberling, J.M.; Miller, J.T.; Noesgaard, D.; Weingart, S.B.; Schigel, D. Data integration enables global biodiversity synthesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2018093118. [Google Scholar] [CrossRef]
- Piccini, I.; Pittarello, M.; Gili, F.; Dotta, A.; Lorizzo, R.; Magnani, C.; Grieco, P.; Lonati, M.; Bertolino, S.; Bonelli, S. Using Forest Compensation Funds to Reverse Biodiversity Loss: A Case Study of Turin–Lyon High-Speed Railway Line. Sustainability 2022, 14, 4411. [Google Scholar] [CrossRef]
- Infusino, M.; Brehm, G.; Di Marco, C.; Scalercio, S. Assessing the efficiency of UV LEDs as light sources for sampling the diversity of macro-moth. Eur. J. Entomol. 2017, 114, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Scalercio, S.; Greco, S. Heterocera fauna of the Calabrian black pine forest, Sila Massif (Italy) (Insecta: Lepidoptera). SHILAP Revta. Lepid. 2018, 46, 455–472. [Google Scholar]
- Parenzan, P.; De Marzo, L. Una nuova trappola luminosa per la raccolta di Lepidotteri e altri insetti ad attività notturna. L’informatore Del Giov. Entomol. Suppl. Al Boll. Della Soc. Entomol. Ital. 1981, 99, 5–11. [Google Scholar]
- Merckx, T.; Slade, E.M. Macro-moth families differ in their attraction to light: Implications for light-trap monitoring programmes. Insect Conserv. Divers. 2014, 7, 453–461. [Google Scholar] [CrossRef]
- Karsholt, O.; Nieukerken, E.V.; Jong, Y.D. Lepidoptera, moths. Fauna Eur. Vers. 2013. [Google Scholar] [CrossRef] [Green Version]
- Frazer, G.W.; Canham, C.D.; Lertzman, K.P. Gap Light Analyzer (GLA), Version 2.0: Imaging Software to Extract Canopy Structure and Gap Light Transmission Indices from True-Colour Fisheye Photographs, User’s Manual and Program Documentation; Simon Fraser University, Burnaby, British Columbia, and the Institute of Ecosystem Studies: New York, NY, USA, 1999; p. 36. [Google Scholar]
- Piccini, I.; Pittarello, M.; Di Pietro, V.; Lonati, M.; Bonelli, S. New approach for butterfly conservation through local field based vegetational and entomological data. Ecosphere 2022, 13, e4026. [Google Scholar] [CrossRef]
- Mironov, V. The Geometrid Moths of Europe. Larentiinae II (Perizomini and Eupitheciini); Apollo Books: Stenstrup, Denmark, 2003; Volume 4, p. 464. [Google Scholar]
- Ghiliani, V. Materiali per Servire alla Compilazione della Fauna Entomologica Italiana, Ossia Elenco delle Specie di Lepidotteri Riconosciute Esistenti Negli Stati Sardi; Memorie Regia Accademia delle scienze di Torino: Turin, Italy, 1852; pp. 131–247. [Google Scholar]
- Hellmann, F.; Parenzan, P.; Balletto, E.; Perosino, G.C.; Mondino, G.P.; Sindaco, R. I macrolepidotteri del Piemonte; Museo regionale di scienze naturali: Turin, Italy, 2010; p. 1058. [Google Scholar]
- Dulaurent, A.M.; Porté, A.J.; van Halder, I.; Vétillard, F.; Menassieu, P.; Jactel, H. A case of habitat complementation in forest pests: Pine processionary moth pupae survive better in open areas. For. Ecol. Manag. 2011, 261, 1069–1076. [Google Scholar] [CrossRef]
- Parisi, F.; Lombardi, F.; Marziliano, P.A.; Russo, D.; De Cristofaro, A.; Marchetti, M.; Tognetti, R. Diversity of saproxylic beetle communities in chestnut agroforestry systems. Iforest-Biogeosci. For. 2020, 13, 456–465. [Google Scholar] [CrossRef]
- Trizzino, M.; Bisi, F.; Morelli, C.E.; Preatoni, D.G.; Wauters, L.A.; Martinoli, A. Spatial niche partitioning of two saproxylic sibling species (Coleoptera, Cetoniidae, genus Gnorimus). Insect Conserv. Divers. 2014, 7, 223–231. [Google Scholar] [CrossRef]
- Beshkov, S. Biodiversity of butterflies and moths (Insecta: Lepidoptera: Macrolepidoptera) in chestnut forests, Belassitsa Mountain (Preliminary report). Project BG 0031 EEA Rep. 2011, 1–18. [Google Scholar]
- Infusino, M.; Greco, S.; Impieri, A.; Scalercio, S. I Macrolepidotteri notturni dei castagneti della Catena Costiera Paolana (Calabria, Italia) (Lepidoptera). Rivista del Museo civico di Scienze Naturali “E. Caffi” Bergamo 2018, 31, 89–134. [Google Scholar]
- Kozár, F.; Benedicty, Z.K.; Fetykó, K.; Kiss, B.; Szita, É. An annotated update of the scale insect checklist of Hungary (Hemiptera, Coccoidea). ZooKeys 2013, 309, 49–66. [Google Scholar]
- Piccini, I.; Caprio, E.; Palestrini, C.; Rolando, A. Ecosystem functioning in relation to species identity, density, and biomass in two tunneller dung beetles. Ecol. Entomol. 2020, 45, 311–320. [Google Scholar] [CrossRef]


| Patches | Elevation m a.s.l. | Slope (°) | Clearing Area (m2) | Canopy Cover (%) | Longitude | Latitude |
|---|---|---|---|---|---|---|
| Clearing 2 | 765.50 | 32.69 | 250.81 | 54.31 | 6.99101645 | 45.1326729 |
| Clearing 3 | 769.30 | 31.07 | 268.97 | 48.85 | 6.9913708 | 45.1332286 |
| Clearing 4 | 770.01 | 40.52 | 324.49 | 53.04 | 6.99142112 | 45.1337146 |
| Clearing 5 | 796.38 | 33.91 | 108.05 | 59.15 | 6.99184302 | 45.1339795 |
| Clearing 7 | 852.24 | 39.51 | 240.88 | 51.68 | 6.99283393 | 45.134654 |
| Clearing 8 | 879.66 | 25.90 | 215.31 | 62.85 | 6.99324809 | 45.1348751 |
| Clearing 9 | 914.83 | 29.37 | 235.95 | 60.96 | 6.99361194 | 45.1354649 |
| Clearing 10 | 944.25 | 34.92 | 218.41 | 44.69 | 6.99390611 | 45.1358527 |
| Clearing 11 | 982.85 | 12.60 | 670.03 | 31.01 | 6.99400307 | 45.1364854 |
| Forest 2 | 764.71 | 21.66 | 82.8 | 6.99193442 | 45.1324905 | |
| Forest 3 | 782.41 | 19.32 | 74.34 | 6.99253588 | 45.1331139 | |
| Forest 4 | 794.26 | 23.67 | 76.94 | 6.99241202 | 45.1335262 | |
| Forest 5 | 816.85 | 27.52 | 82.53 | 6.99276425 | 45.1338266 | |
| Forest 7 | 850.16 | 27.57 | 88.64 | 6.99396418 | 45.1344437 | |
| Forest 8 | 873.66 | 29.16 | 86.07 | 6.99416932 | 45.1348506 | |
| Forest 9 | 909.14 | 39.27 | 83.07 | 6.99455639 | 45.1354704 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccini, I.; Depetris, M.; Paradiso, F.; Cochis, F.; Audisio, M.; Artioli, P.; Smargiassi, S.; Bonifacino, M.; Giuliano, D.; La Cava, S.; et al. Macro-moth (Lepidoptera) Diversity of a Newly Shaped Ecological Corridor and the Surrounding Forest Area in the Western Italian Alps. Diversity 2023, 15, 95. https://doi.org/10.3390/d15010095
Piccini I, Depetris M, Paradiso F, Cochis F, Audisio M, Artioli P, Smargiassi S, Bonifacino M, Giuliano D, La Cava S, et al. Macro-moth (Lepidoptera) Diversity of a Newly Shaped Ecological Corridor and the Surrounding Forest Area in the Western Italian Alps. Diversity. 2023; 15(1):95. https://doi.org/10.3390/d15010095
Chicago/Turabian StylePiccini, Irene, Marta Depetris, Federica Paradiso, Francesca Cochis, Michela Audisio, Patrick Artioli, Stefania Smargiassi, Marco Bonifacino, Davide Giuliano, Sara La Cava, and et al. 2023. "Macro-moth (Lepidoptera) Diversity of a Newly Shaped Ecological Corridor and the Surrounding Forest Area in the Western Italian Alps" Diversity 15, no. 1: 95. https://doi.org/10.3390/d15010095