A Unified Approach to Analysis of Body Condition in Green Toads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Data Acquisition
2.3. Statistical Analysis
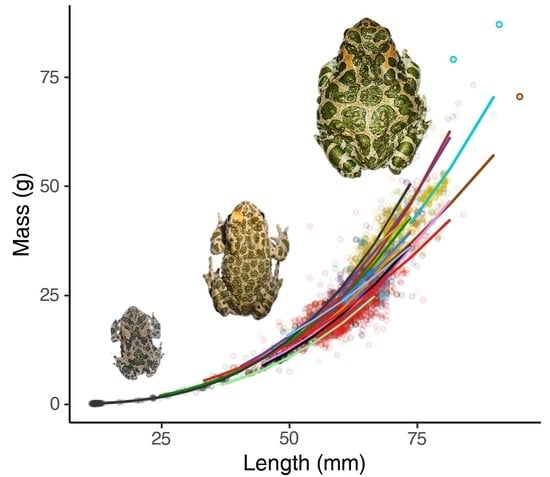
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Green, A.J. Mass/Length Residuals: Measures of Body Condition or Generators of Spurious Results? Ecology 2001, 82, 1473–1483. [Google Scholar] [CrossRef]
- Reading, C.J. Linking Global Warming to Amphibian Declines through Its Effects on Female Body Condition and Survivorship. Oecologia 2007, 151, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Boyero, L.; López-Rojo, N.; Bosch, J.; Alonso, A.; Correa-Araneda, F.; Pérez, J. Microplastics Impair Amphibian Survival, Body Condition and Function. Chemosphere 2020, 244, 125500. [Google Scholar] [CrossRef] [PubMed]
- Guerra, C.; Aráoz, E. Amphibian Malformations and Body Condition across an Agricultural Landscape of Northwest Argentina. Dis. Aquat. Org. 2016, 121, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Peig, J.; Green, A.J. The Paradigm of Body Condition: A Critical Reappraisal of Current Methods Based on Mass and Length: The Paradigm of Body Condition. Funct. Ecol. 2010, 24, 1323–1332. [Google Scholar] [CrossRef]
- Jakob, E.M.; Marshall, S.D.; Uetz, G.W. Estimating Fitness: A Comparison of Body Condition Indices. Oikos 1996, 77, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Băncilă, R.I.; Hartel, T.; Plăiaşu, R.; Smets, J.; Cogălniceanu, D. Comparing Three Body Condition Indices in Amphibians: A Case Study of Yellow-Bellied Toad Bombina variegata. Amphib.-Reptil. 2010, 31, 558–562. [Google Scholar] [CrossRef]
- Peig, J.; Green, A.J. New Perspectives for Estimating Body Condition from Mass/Length Data: The Scaled Mass Index as an Alternative Method. Oikos 2009, 118, 1883–1891. [Google Scholar] [CrossRef]
- MacCracken, J.G.; Stebbings, J.L. Test of a Body Condition Index with Amphibians. J. Herpetol. 2012, 46, 346–350. [Google Scholar] [CrossRef]
- Drakulić, S.; Feldhaar, H.; Lisičić, D.; Mioč, M.; Cizelj, I.; Seiler, M.; Spatz, T.; Rödel, M. Population-specific Effects of Developmental Temperature on Body Condition and Jumping Performance of a Widespread European Frog. Ecol. Evol. 2016, 6, 3115–3128. [Google Scholar] [CrossRef]
- Dittrich, C.; Drakulić, S.; Schellenberg, M.; Thein, J.; Rödel, M.-O. Some like It Hot? Developmental Differences in Yellow-Bellied Toad (Bombina variegata) Tadpoles from Geographically Close but Different Habitats. Can. J. Zool. 2016, 94, 69–77. [Google Scholar] [CrossRef]
- Courant, J.; Secondi, J.; Bereiziat, V.; Herrel, A. Resources Allocated to Reproduction Decrease at the Range Edge of an Expanding Population of an Invasive Amphibian. Biol. J. Linn. Soc. 2017, 122, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Gould, J.; Valdez, J.W. Dating on Your Level: Assortative Mating Based on Body Condition in an Amphibian. Ethol. Ecol. Evol. 2021, 1–15. [Google Scholar] [CrossRef]
- Rosa, G.; Costa, A.; Renet, J.; Romano, A.; Roner, L.; Salvidio, S. Energy Storage in Salamanders’ Tails: The Role of Sex and Ecology. Sci. Nat. 2021, 108, 27. [Google Scholar] [CrossRef]
- Roth, P. Bufo viridis Laurenti, 1768. In Atlas of Amphibians and Reptiles in Europe; Muséum National d’Histoire Naturelle: Paris, France, 1997; pp. 122–123. [Google Scholar]
- Heigl, F.; Horvath, K.; Laaha, G.; Zaller, J.G. Amphibian and Reptile Road-Kills on Tertiary Roads in Relation to Landscape Structure: Using a Citizen Science Approach with Open-Access Land Cover Data. BMC Ecol. 2017, 17, 24. [Google Scholar] [CrossRef]
- Valkanova, M.V.; Mollov, I.A.; Nikolov, B.N. Mortalities of the Green Toad, Epidalea viridis (Laurenti, 1768) in Urban Environment: A Case Study from the City of Plovdiv. Ecol. Balk. 2009, 1, 21–26. [Google Scholar]
- Vences, M. Development of New Microsatellite Markers for the Green Toad, Bufotes viridis, to Assess Population Structure at Its Northwestern Range Boundary in Germany. Salamandra 2019, 55, 191–198. [Google Scholar] [CrossRef]
- Mazgajska, J.; Mazgajski, T.D. Two Amphibian Species in the Urban Environment: Changes in the Occurrence, Spawning Phenology and Adult Condition of Common and Green Toads. Eur. Zool. J. 2020, 87, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek, J.M.; Kaczmarski, M.; Pedziwiatr, K. Changes in the Batrachofauna in the City of Poznań over 20 Years. In Animal, Man, and the City–Interactions and Relationships; Uniwersytet Techniczno-Przyrodniczy: Bydgoszcz, Poland, 2014. [Google Scholar]
- Konowalik, A.; Najbar, A.; Konowalik, K.; Dylewski, Ł.; Frydlewicz, M.; Kisiel, P.; Starzecka, A.; Zaleśna, A.; Kolenda, K. Amphibians in an Urban Environment: A Case Study from a Central European City (Wrocław, Poland). Urban Ecosyst. 2020, 23, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Dufresnes, C.; Mazepa, G.; Jablonski, D.; Oliveira, R.C.; Wenseleers, T.; Shabanov, D.A.; Auer, M.; Ernst, R.; Koch, C.; Ramírez-Chaves, H.E.; et al. Fifteen Shades of Green: The Evolution of Bufotes Toads Revisited. Mol. Phylogenet. Evol. 2019, 141, 106615. [Google Scholar] [CrossRef]
- Speybroeck, J.; Beukema, W.; Dufresnes, C.; Fritz, U.; Jablonski, D.; Lymberakis, P.; Martínez-Solano, I.; Razzetti, E.; Vamberger, M.; Vences, M.; et al. Species List of the European Herpetofauna–2020 Update by the Taxonomic Committee of the Societas Europaea Herpetologica. Amphib.-Reptil. 2020, 41, 139–189. [Google Scholar] [CrossRef]
- Sinsch, U.; Leskovar, C. Does Thermoregulatory Behaviour of Green Toads (Bufo viridis) Constrain Geographical Range in the West? A Comparison with the Performance of Syntopic Natterjacks (Bufo calamita). J. Therm. Biol. 2011, 36, 346–354. [Google Scholar] [CrossRef]
- Sistani, A.; Burgstaller, S.; Gollmann, G.; Landler, L. The European Green Toad, Bufotes viridis, in Donaufeld (Vienna, Austria): Status and Size of the Population. Herpetozoa 2021, 34, 259–264. [Google Scholar] [CrossRef]
- Andrä, E. Höchstgelegenes Laichhabitat der Wechselkröte (Bufo viridis) in den Bayerischen Voralpen und Zusammenstellung der Fundpunkte der Art im Grenzbereich von Bayern und Österreich. Z. Feldherpetol. 1999, 6, 187–2002. [Google Scholar]
- Andrä, E.; Deuringer-Andrä, M. Höchstgelegenes Laichhabitat der Wechselkröte (Bufo viridis) in Mitteleuropa nördlich des Alpenhauptkammes im Grenzbereich zwischen Bayern und Tirol. Z. Feldherpetol. 2011, 18, 19–68. [Google Scholar]
- Ott, M. Telemetriestudie Zur Raum- und Habitatnutzung der Wechselkröte (Bufotes variabilis PALLAS, 1769) im Sommerlebensraum auf der Ostseeinsel Fehmarn. Master’s Thesis, Universität für Bodenkultur, Vienna, Austria, 2015. [Google Scholar]
- Kuhn, W. Kreuzkröte (Bufo calamita) und Wechselkröte (Bufo viridis): Eine Mischpopulation am südlichen Rand ihres Verbreitungsgebietes in Bayern. Ph.D. Thesis, Landnutzung und Umwelt der Technischen Universität München, Munich, Germany, 2000. [Google Scholar]
- Mühlbauer, M.; Zahn, A.; Köbele, C.; Sedlmeier, H. Manche mögen’s heiß: Verstecke und Lebensräume junger Wechselkröten (Bufotes viridis). Z. Feldherpetol. 2015, 22, 191–210. [Google Scholar]
- Zhelev, Z.; Mollov, I.; Tsonev, S. Body Size and Color Polymorphism in Bufotes viridis Complex (Anura: Bufonidae) Inhabiting Two Semi-Natural Areas in Plovdiv City, Bulgaria. North-West. J. Zool. 2020, 16, 191–196. [Google Scholar]
- Mazgajska, J.; Mazgajski, T.D. Low Recapture Rate in PIT Marked Urban Populations of the Common Toad. Pol. J. Ecol. 2016, 64, 586–593. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012; ISBN 3-90-005107-0. [Google Scholar]
- Yu, C.; Yao, W. Robust Linear Regression: A Review and Comparison. Commun. Stat. Simul. Comput. 2017, 46, 6261–6282. [Google Scholar] [CrossRef]
- Liao, C.-P. An R Function: OLS/Robust Scaled Mass Index. Apan’s Notes 2018. Available online: http://apansharing.blogspot.com/2018/05/an-r-function-olsrobust-caled-mass-index.html (accessed on 9 December 2022).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin, Germany, 2016. [Google Scholar]
- Lüdecke, D. Ggeffects: Tidy Data Frames of Marginal Effects from Regression Models. J. Open Source Softw. 2018, 3, 772. [Google Scholar] [CrossRef] [Green Version]
- Kassambara, A. Ggpubr: “ggplot2” Based Publication Ready Plots; 2020. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 9 December 2022).
- Hollister, J.W. Elevatr: Access Elevation Data from Various APIs; R Package Version 0.4.1. 2021. Available online: https://CRAN.R-project.org/package=elevatr/ (accessed on 9 December 2022).
- Iannone, R.; Cheng, J.; Schloerke, B.; Hughes, E. Gt: Easily Create Presentation-Ready Display Table; R Package Version 0.7.0. 2022. Available online: https://CRAN.R-project.org/package=gt (accessed on 9 December 2022).
- Santini, L.; Benítez-López, A.; Ficetola, G.F.; Huijbregts, M.A.J. Length–Mass Allometries in Amphibians. Integr. Zool. 2018, 13, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Brodeur, J.C.; Vera Candioti, J.; Damonte, M.J.; Bahl, M.F.; Poliserpi, M.B.; D’Andrea, M.F. Frog Somatic Indices: Importance of Considering Allometric Scaling, Relation with Body Condition and Seasonal Variation in the Frog Leptodactylus latrans. Ecol. Indic. 2020, 116, 106496. [Google Scholar] [CrossRef]
- Estrada, A.; Medina, D.; Gratwicke, B.; Ibáñez, R.; Belden, L.K. Body Condition, Skin Bacterial Communities and Disease Status: Insights from the First Release Trial of the Limosa Harlequin Frog, Atelopus limosus. Proc. R. Soc. B 2022, 289, 20220586. [Google Scholar] [CrossRef]
- Castellano, S.; Cucco, M.; Giacoma, C. Reproductive Investment of Female Green Toads (Bufo viridis). Copeia 2004, 2004, 659–664. [Google Scholar] [CrossRef]
- Baumgartner, M.; Gollmann, G. Conservation of the Yellow-Bellied Toad in Gesäuse National Park: Collecting Baseline Data. In Proceedings of the 6th Symposium for Research in Protected Areas, Salzburg, Austria, 2–3 November 2017; pp. 41–43. [Google Scholar]
- Falk, B.G.; Snow, R.W.; Reed, R.N. A Validation of 11 Body-Condition Indices in a Giant Snake Species That Exhibits Positive Allometry. PLoS ONE 2017, 12, e0180791. [Google Scholar] [CrossRef]
- Höglund, J.; Bolender, L.; Cortazar-Chinarro, M.; Meurling, S.; Laurila, A.; Hermaniuk, A.; Dufresnes, C. Low Neutral and Immunogenetic Diversity in Northern Fringe Populations of the Green Toad Bufotes viridis: Implications for Conservation. Conserv. Genet. 2022, 23, 139–149. [Google Scholar] [CrossRef]



| Population | Latitude | Longitude | Elevation | Sample Size (n) | Population-Specific Coefficient | Length in mm (Mean) | Length in mm (Range) | Mass in g (Mean) | Mass in g (Range) | SMI Pop. Specific (Mean) | SMI Pop. Specific (SD) | SMI.gt (Mean) | SMI.gt (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fehmarn Island (DE) | 54.4518 | 11.0426 | 1 | 19 | 2.349 | 73.8 | 62–95 | 37.5 | 27.2–70.6 | 22.8 | 1.9 | 20.0 | 2.3 |
| Warsaw (PL) | 52.2676 | 21.0008 | 92 | 78 | 3.128 | 65.7 | 51–81 | 29.0 | 13.7–61.4 | 21.4 | 2.8 | 21.6 | 2.8 |
| Esser Kieswerke (DE) | 51.0113 | 6.8690 | 39 | 12 | 3.233 | 62.0 | 54.8–66.2 | 21.8 | 15.7–27.1 | 19.5 | 1.5 | 19.6 | 1.4 |
| Ginsterpfad (DE) | 50.9858 | 6.9315 | 42 | 20 | 2.763 | 65.3 | 58.3–73.6 | 25.3 | 19.6–34.2 | 19.8 | 2.0 | 19.5 | 2.0 |
| Westhoven (DE) | 50.9030 | 7.0106 | 45 | 24 | 2.747 | 66.2 | 44.4–82.7 | 28.9 | 11.93–66.4 | 21.0 | 3.5 | 20.4 | 4.1 |
| Porz-Wahn (DE) | 50.8631 | 7.0896 | 54 | 23 | 4.198 | 65.6 | 56.1–76.6 | 25.4 | 15.9–38.63 | 17.3 | 3.2 | 19.2 | 3.3 |
| Basell (DE) | 50.8591 | 6.9485 | 46 | 33 | 3.559 | 63.2 | 34.6–72.2 | 21.9 | 3.95–31.9 | 17.7 | 2.6 | 18.2 | 2.0 |
| Urmitz (DE) | 50.4081 | 7.5251 | 66 | 209 | 2.973 | 58.5 | 41–76 | 20.2 | 6.6–64.2 | 20.7 | 3.1 | 20.7 | 3.1 |
| Donaufeld (AT) | 48.2499 | 16.4196 | 161 | 68 | 2.689 | 69.8 | 40–86 | 41.2 | 8.5–73.2 | 27.1 | 3.7 | 25.9 | 3.7 |
| Bednar Park (AT) | 48.2258 | 16.3972 | 161 | 178 | 2.853 | 58.0 | 23–78 | 23.6 | 1.4–63.4 | 24.4 | 3.9 | 24.4 | 4.0 |
| Simmering (AT) | 48.1690 | 16.4454 | 156 | 840 | 2.816 | 61.1 | 30–84 | 22.4 | 3.5–54.56 | 20.8 | 3.5 | 20.7 | 3.7 |
| Jesenwang (DE) | 48.1680 | 11.1551 | 556 | 131 | 3.522 | 61.8 | 48–83 | 27.5 | 14–69 | 24.1 | 3.2 | 24.4 | 3.1 |
| Riem (DE) | 48.1336 | 11.7000 | 527 | 110 | 3.169 | 27.4 | 11.25–81 | 6.8 | 0.11–65.4 | 26.8 | 3.0 | 22.8 | 3.4 |
| Seewinkel (AT) | 47.7680 | 16.7866 | 116 | 100 | 2.956 | 65.6 | 55–81 | 30.3 | 17.5–49.5 | 22.8 | 3.2 | 22.7 | 3.3 |
| Hochriesgebiet (DE) | 47.7465 | 12.2608 | 1186 | 21 | 2.852 | 76.6 | 67–91 | 47.6 | 30.5–87.2 | 23.3 | 3.3 | 22.5 | 3.2 |
| Plovdiv (BG) | 42.1573 | 24.7433 | 164 | 61 | 3.230 | 67.3 | 49–79.15 | 29.6 | 9–53 | 21.0 | 5.1 | 21.6 | 5.1 |
| Galabovo (BG) | 42.1378 | 25.8670 | 96 | 269 | 2.042 | 71.6 | 61.33–81.12 | 41.1 | 30.58–53.22 | 28.6 | 2.4 | 24.3 | 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landler, L.; Burgstaller, S.; Spießberger, M.; Horvath, A.; Zhelev, Z.; Mollov, I.; Sinsch, U.; Nepita, J.; Schwabel, F.; Kuhn, W.; et al. A Unified Approach to Analysis of Body Condition in Green Toads. Diversity 2023, 15, 43. https://doi.org/10.3390/d15010043
Landler L, Burgstaller S, Spießberger M, Horvath A, Zhelev Z, Mollov I, Sinsch U, Nepita J, Schwabel F, Kuhn W, et al. A Unified Approach to Analysis of Body Condition in Green Toads. Diversity. 2023; 15(1):43. https://doi.org/10.3390/d15010043
Chicago/Turabian StyleLandler, Lukas, Stephan Burgstaller, Magdalena Spießberger, Andras Horvath, Zhivko Zhelev, Ivelin Mollov, Ulrich Sinsch, Johannes Nepita, Florian Schwabel, Wolfgang Kuhn, and et al. 2023. "A Unified Approach to Analysis of Body Condition in Green Toads" Diversity 15, no. 1: 43. https://doi.org/10.3390/d15010043