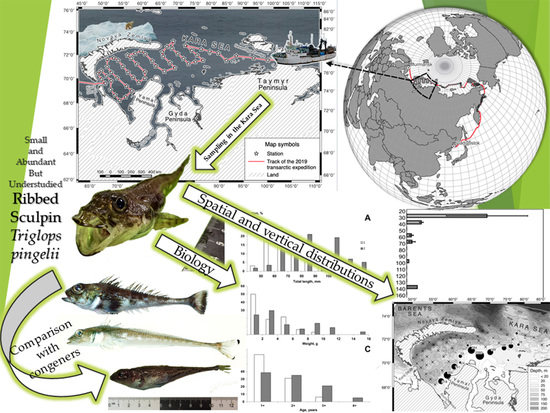
Small and Abundant but Understudied Ribbed Sculpin Triglops pingelii (Cottidae, Teleostei) from the Kara Sea (Siberian Arctic): Distribution, Biology, and Comparison with Congeners
Abstract
:1. Introduction
2. Materials and Methods
2.1. Survey Design
2.2. Biological Sampling
2.3. Data Analysis and Statistics
3. Results and Discussion
3.1. Distribution
3.2. Length and Weight
3.3. Sexual Dimorphism and Fecundity
3.4. Diet Composition and Feeding Habits
3.5. Comparison with Congeners
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knipovich, N.M. Guide to the Fish of the Barents, White and Kara Seas. Tr. Sci. Res. Inst. Stud. North 1926, 27, 1–183. [Google Scholar]
- Schmidt, P.Y. Fish of the Sea of Okhotsk; Publishing House of the USSR Academy of Sciences: Moscow, Russia, 1950; pp. 1–370. [Google Scholar]
- Esipov, V.K. Fishes of the Kara Sea; Publishing House of the USSR Academy of Sciences: Moscow-Leningrad, Russia, 1952; pp. 1–147. [Google Scholar]
- Andriashev, A.P. Fishes of the Northern Seas of the USSR; Israel Program for Scientific Translations: Washington, DC, USA, 1964; pp. 1–617. [Google Scholar]
- Fedorov, V.V. List of Fishes of the Bering Sea. Izv. TINRO 1973, 87, 42–71. [Google Scholar]
- Lindberg, G.U.; Krasyukova, Z.V. Fishes of the Sea of Japan and Adjacent Parts of the Okhotsk and Yellow Seas; Part 5; Nauka: Leningrad, Russia, 1987; pp. 1–526. [Google Scholar]
- Allen, M.J.; Smith, G.B. Atlas and Zoogeography of Common Fishes in the Bering Sea and Northeastern Pacific. U.S. Dept. Commer. NOAA Technol. Rep. NMFS 1988, 66, 1–151. [Google Scholar]
- Andriashev, A.P.; Chernova, N.V. Annotated List of Pisciformes and Fishes of the Seas of the Arctic Region and Adjacent Waters. Vopr. Ikhtiol. 1994, 34, 435–456. [Google Scholar]
- Pietsch, T.W. Systematics and distribution of cottid fishes of the genus Triglops Reinhardt (Teleostei: Scorpaeniformes). Zool. J. Linn. Soc. 1994, 109, 335–393. [Google Scholar] [CrossRef]
- Borets, L.A. Annotated List of Fish of Far Eastern Seas; TINRO-Tsentr: Vladivostok, Russia, 2000; pp. 1–192. [Google Scholar]
- Borets, L.A. Bottom Iichthyocenoses of the Russian Shelf of Far Eastern Seas: Composition, Structure, Elements of Functioning, and Commercial Importance; TINRO-Tsentr: Vladivostok, Russia, 1997; pp. 1–217. [Google Scholar]
- Mecklenburg, C.; Mecklenburg, T.; Thorsteinson, L. Fishes of Alaska; American Fisheries Society: Bethesda, MD, USA, 2002; pp. 1–1037. [Google Scholar]
- Dolgov, A.V. Ichthyofauna Species Composition and Ichthyocenose Structure of the Barents Sea. Izv. TINRO 2004, 137, 177–195. [Google Scholar]
- Dolgov, A.V. Non-Commercial Fish and Skates. In PINRO Research in the Area of the Svalbard Archipelago; PINRO Publishing: Murmansk, Russia, 2004; pp. 229–274. [Google Scholar]
- Datsky, A.V.; Andronov, P.Y. Ichthyocene of the Upper Shelf of the Northwestern Part of the Bering Sea; Sev.-Vost. Nauchn. Tsentr, Dal’nevost. Otd., Ross. Akad. Nauk: Magadan, Russia, 2007; pp. 1–261. [Google Scholar]
- Chereshnev, I.A.; Kirillov, A.F. Fishlike Vertebrates and Fishes from the Laptev Sea and the East-Siberian Sea and Their Related Freshwater Areas. Bull. North East Sci. Center Rus. Acad. Sci. Far East Branch 2007, 2, 95–106. [Google Scholar]
- Parin, N.V.; Evseenko, S.A.; Vasilyeva, E.D. Fishes of the Seas of Russia: Annotated Catalog; KMK Press: Moscow, Russia, 2014; pp. 1–733. [Google Scholar]
- Tuponogov, V.N.; Kodolov, L.S. Handbook for Identification of the Commercial and Mass Species of Fishes of Far Eastern Seas of Russia; Russkiy Ostrov: Vladivostok, Russia, 2014; pp. 1–336. [Google Scholar]
- Tuponogov, V.N.; Yavnov, S.V. Atlas of Fish of the Far Eastern Seas of Russia (Rockfishes, Greenlings, Sculpins, Sea Poachers); Russkiy Ostrov: Vladivostok, Russia, 2015; pp. 1–264. [Google Scholar]
- Kirillov, A.F.; Apsolikhova, O.D.; Zhirkov, F.N.; Karpova, L.N.; Sveshnikov, Y.A.; Burmistrov, E.V. Annotated List of Fish-like and Fishes of the East Siberian Sea Basin. Stud. Aquat. Biol. Resour. Kamchatka Northw. Pac. Ocean 2016, 42, 78–87. [Google Scholar]
- Orlov, A.M.; Benzik, A.N.; Rybakov, M.O.; Nosov, M.A.; Gorbatenko, K.M.; Vedishcheva, E.V.; Orlova, S.Y. Some Preliminary Results of Biological Studies in the Kara Sea at RV «Professor Levanidov» in September 2019. Tr. VNIRO 2020, 182, 201–215. [Google Scholar] [CrossRef]
- Sheiko, B.A.; Fedorov, V.V. Class Cephalaspidomorphi—Lampreys. Class Chondrichthyes—Cartilaginous Fishes. Class Holocephali—Chimaeras. Class Osteichthyes—Bony Fishes. In Catalogue of Vertebrates of Kamchatka and Adjacent Waters; Kamchatskii Pechatnyi Dvor: Petropavlovsk-Kamchatsky, Russia, 2000; pp. 7–69. [Google Scholar]
- Novikov, N.P.; Sokolovsky, A.S.; Sokolovskaya, T.G.; Yakovlev, Y.M. Fishes of Primorye; Dalrybvtuz: Vladivostok, Russia, 2002; pp. 1–552. [Google Scholar]
- Wienerroither, R.; Johannesen, E.; Dolgov, A.; Byrkjedal, I.; Bjelland, O.; Drevetnyak, K.; Eriksen, K.B.; Høines, Å.; Langhelle, G.; Langøy, H.; et al. Atlas of the Barents Sea Fishes. IMR/PINRO Jt. Rept. Ser. 2011, 1, 1–274. [Google Scholar]
- Dolgov, A.V.; Novoselov, A.P.; Prokhorova, T.A.; Fuks, G.V.; Prozorkevich, D.V.; Chernova, N.V.; Sherstkov, V.S.; Levitskiy, A.L. Atlas of the Kara Sea Fish; PINRO: Murmansk, Russia, 2018; pp. 1–271. [Google Scholar]
- Dolgov, A.V.; Smirnov, O.V.; Sentyabov, E.V.; Drevetnyak, K.M.; Chetyrkina, O.Y. New Data on the Ichthyofauna of the Kara Sea (Based on the Results of PINRO Studies in 2007–2008). In Terrestrial and Marine Ecosystems; Paulsen LLC: Moscow, Russia, 2011; pp. 112–128. [Google Scholar]
- Prishchepa, B.F. (Ed.) Ecosystem of the Kara Sea; PINRO: Murmansk, Russia, 2008; pp. 1–261. [Google Scholar]
- Dolgov, A.V. Some Features of the Biology of Non-Commercial Fish of the Barents Sea. In Problems of Fisheries Science in the Work of the Young: Collected Papers of the Conference-Contest of PINRO Young Scientists and Specialists; PINRO Publishing: Murmansk, Russia, 1995; pp. 69–94. [Google Scholar]
- Dolgov, A.V. Composition, Formation, and Trophic Structure of the Ichthyocene of the Barents Sea. Ph.D. Thesis, VNIRO, Moscow, Russia, 2012; pp. 1–48. [Google Scholar]
- Dolgov, A.V. Feeding of Non-commercial Fish in the Northern Barents Sea. In Features of the Formation of Bioproductivity of the Northern Regions of the Barents Sea during the Warming of the Arctic: Collected Papers; PINRO Publishing: Murmansk, Russia, 2014; pp. 155–185. [Google Scholar]
- Bogdanova, V.A.; Bondarev, O.V. Some Aspects of the Biology of the Non-Commercial Species of the Kara Sea Ichthyofauna, Triglops pingelii. In Problems of the Arctic Region: Abstracts of the XVIII International Scientific Conference of Students and Postgraduates (Murmansk, May 15, 2019); KNC RAS: Murmansk, Russia, 2019; pp. 31–32. [Google Scholar]
- Orlov, A.M.; Savin, A.B.; Gorbatenko, K.M.; Benzik, A.N.; Morozov, T.B.; Rybakov, M.O.; Terentiev, D.A.; Vedishcheva, E.V.; Kurbanov, Y.K.; Nosov, M.A.; et al. Biological Studies in the Russian Far Eastern and Arctic Seas. Tr. VNIRO 2020, 181, 102–143. [Google Scholar] [CrossRef]
- Orlov, A.M.; Gorbatenko, K.M.; Benzik, A.N.; Rybakov, M.O.; Nosov, M.A.; Orlova, S.Y. Biological Research in the Siberian Arctic Seas in Summer–Autumn 2019 (Cruise of the R/V Pprofessor Levanidov). Oceanology 2021, 61, 295–296. [Google Scholar] [CrossRef]
- Orlov, A.M.; Rybakov, M.O.; Vedishcheva, E.V.; Volkov, A.A.; Orlova, S.Y. Walleye Pollock Gadus chalcogrammus, a Species with Continuous Range from the Norwegian Sea to Korea, Japan, and California: New Records from the Siberian Arctic. J. Mar. Sci. Engin. 2021, 9, 1141. [Google Scholar] [CrossRef]
- Tokranov, A.M.; Emelin, P.O.; Orlov, A.M. Small but Abundant: Distribution and Biology of Arctic Staghorn Sculpin Gymnocanthus tricuspis (Cottidae) in the Kara Sea. J. Ichthyol. 2022, 62, 885–899. [Google Scholar] [CrossRef]
- Dolgov, A.V. Annotated List of Fish-Like Vertebrates and Fish of the Kara Sea. J. Ichthyol. 2013, 53, 914–922. [Google Scholar] [CrossRef]
- Laevatsu, T. Manual of Methods in Fishery Biology. FAO Manuals in Fisheries Science; FAO: Rome, Italy, 1965; pp. 1–51. [Google Scholar]
- Pravdin, I.F. Guide to the Study of Fish; Pishchevaya Promyshlennost’: Moscow, Russia, 1966; pp. 1–374. [Google Scholar]
- Tokranov, A.M.; Orlov, A.M. Some Biological Features of Rare and Poorly Studied Sculpins (Fam. Cottidae, Hemitripteridae, Psychrolutidae) in the Pacific Waters off the Northern Kuril Islands and Southeastern Kamchatka. Raffles Bull. Zool. 2007, (Suppl. S14), 171–182. [Google Scholar]
- Anonymous. Manual for Analysis of Feeding and Food Relations of Fishes in Natural Conditions; Nauka: Moscow, Russia, 1974; pp. 1–254. [Google Scholar]
- Schumann, A.H. Thiessen Polygon. Encyclopedia of Hydrology and Lakes. Encyclopedia of Earth Science; Springer: Dordrecht, The Netherlands, 1998. [Google Scholar] [CrossRef]
- QGIS. QGIS 3.16 Documentation. 24.1.16.81. Voronoi Polygons. 1981. Available online: https://docs.qgis.org/3.16/en/docs/user_manual/processing_algs/qgis/vectorgeometry.html?highlight=voronoi#voronoi-polygons (accessed on 8 December 2021).
- Jakobsson, M.; Mayer, L.A.; Coakley, B.; Dowdeswell, J.A.; Forbes, S.; Fridman, B.; Hodnesdal, H.; Noormets, R.; Pedersen, R.; Rebesco, M.; et al. The International Bathymetric Chart of the Arctic Ocean (IBCAO). Version 3.0. Geophys. Res. Lett. 2012, 39, L12609. [Google Scholar] [CrossRef] [Green Version]
- Mularie, W.M. World Geodetic System 1984—Its Definition and Relationships with Local Geodetic Systems. In Technol. Rep. TR8350.2; National Imagery and Mapping Agency: St. Louis, MO, USA, 2000. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Tokranov, A.M.; Orlov, A.M. Specific Distribution and Catch Dynamics of Cottidae Fishes of Genus Triglops Reinhardt, 1830 (Cottidae) in Pacific Waters of Northern Kuril Islands and Southeastern Kamchatka. In Conservation of Biological Diversity of Kamchatka and Adjacent Seas: Proceedings of the IX International Scientific Conference; Kamchatpress: Petropavlovsk-Kamchatsky, Russia, 2008; pp. 125–140. [Google Scholar]
- Pushchina, O.I.; Panchenko, V.V.; Boyko, M.I.; Galeev, A.I. Distribution and Some Traits of Biology of Sculpins of genus Triglops (Cottidae) in the Sea of Japan. J. Ichthyol. 2021, 61, 130–142. [Google Scholar] [CrossRef]
- Chernova, N.V. Ichthyofauna of Marine Waters of Novosibirskie Islands (Protected Area of the Ust–Lensky Nature Reserve). Nauch. Tr. Gos. Prirod. Zapoved. Prisurskii 2015, 30, 271–276. [Google Scholar]
- Tokranov, A.M. The Size and Sex structure of Cottid Fishes of the Genus Triglops (Cottidae) in the Coastal Waters of Kamchatka. Vopr. Ichthyol. 1995, 35, 134–136. [Google Scholar]
- Tokranov, A.M. On Sexual Dimorphism in Cottid Fishes (Cottidae, Pisces) of the Kamchatka Waters. In Modern Problems of Evolution and Ecology, Proceedings of the International Conference “XXX Lyubishchevskie Readings—2016”, Ulyanovsk, Russia, 5–7 April 2016; Ulyanovsk State Pedagogical University: Ulyanovsk, Russia, 2016; pp. 124–131. [Google Scholar]
- Froese, R. Cube Law, Condition Factor and Weight–Length Relationships: History, Meta-Analysis and Recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Dutta, S.; Orlov, A.; Hazra, S. Population Biology and Exploitation Status of Four Commercially Important Marine Fishes of the Northern Bay of Bengal, India. Iran. J. Fish. Sci. 2021, 20, 62–83. [Google Scholar] [CrossRef]
- Orlov, A.M.; Mishin, A.V.; Artemenkov, D.V.; Murzina, S.A. Length-weight characteristics of some pelagic fishes in the high latitudes of Atlantic sector of the Southern Ocean. J. Ichthyol. 2022, 62. in press. [Google Scholar]
- Tokranov, A.M. Feeding Habits of Cottid Fishes of the Genus Triglops Reinhardt (Cottidae) in the Coastal Waters of Kamchatka. Bull. Mos. Soc. Nat. Dept. Biol. 1991, 96, 46–52. [Google Scholar]
- Tokranov, A.M. Trophic Groups of the Sculpins (Cottidae) in the Waters Near Kamchatka. Principy Ecol. 2019, 8, 123–132. [Google Scholar] [CrossRef]
- Tokranov, A.M. Trophic Groupings of Benthic and Bentho-pelagic Fish of Various Families of the Order Scorpaeniformes in the Kamchatka Waters. In Proceedings of the Aquatic Bioresources, Aquaculture and Ecology of Reservoirs: Materials of the VIII International Baltic Sea Forum, Kaliningrad, Russia, 5–10 October 2020; Kaliningrad State Technical University: Kaliningrad, Russia, 2020; Volume 3, pp. 107–117. [Google Scholar]
- Chuchukalo, V.I. Feeding and Trophic Relations of Nekton and Nektobenthos in Far Eastern Seas; TINRO–Tsentr: Vladivostok, Russia, 2006; pp. 1–484. [Google Scholar]
- Musick, J.A.; Able, K.W. Occurrence and Spawning of the Sculpin Triglops murrayi (Pisces, Cottidae) in the Gulf of Maine. J. Fish. Res. Bd. Can. 1969, 26, 473–475. [Google Scholar] [CrossRef]
- Ryzhkov, L.P.; Trofimov, I.I. Some Ecological and Biological Data on the Bigeye Sculpin (Triglops nybelini) of the Barents Sea. Uch. Zap. Petrozavodsk. State Univ. 2013, 6, 7–9. [Google Scholar]









| Station No. | Area Surveyed, km2 | Station No. | Area Surveyed, km2 | Station No. | Area Surveyed, km2 |
|---|---|---|---|---|---|
| 1 | 12,458.7 | 21 | 7889.715 | 41 | 4833.349 |
| 2 | 22,776.87 | 22 | 13,315.62 | 42 | 5914.237 |
| 3 | 22,059.71 | 23 | 9364.74 | 43 | 8954.832 |
| 4 | 24,801.19 | 24 | 4917.381 | 44 | 8711.212 |
| 5 | 23,856.32 | 25 | 11,867.81 | 45 | 3542.206 |
| 6 | 11,116.17 | 26 | 9724.985 | 46 | 8435.111 |
| 7 | 5257.836 | 27 | 4142.302 | 47 | 5220.943 |
| 8 | 7304.257 | 28 | 5233.781 | 48 | 8974.715 |
| 9 | 8559.135 | 29 | 7917.345 | 49 | 7329.788 |
| 10 | 5178.299 | 30 | 5569.498 | 50 | 5132.056 |
| 11 | 29,960.82 | 31 | 9090.005 | 51 | 3593.312 |
| 12 | 15,076.19 | 32 | 8713.412 | 52 | 4411.633 |
| 13 | 18,821.87 | 33 | 6815.545 | 53 | 2772.137 |
| 14 | 10,924.8 | 34 | 7226.885 | 54 | 4257.445 |
| 15 | 10,005.91 | 35 | 8747.315 | 55 | 4916.166 |
| 16 | 6831.042 | 36 | 10,485.36 | ||
| 17 | 5243.73 | 37 | 7916.764 | ||
| 18 | 8998.251 | 38 | 9058.273 | ||
| 19 | 8374.541 | 39 | 6369.133 | ||
| 20 | 6924.034 | 40 | 5384.944 | ||
| Total | 55 | 511,279.636 | |||
| Parameter | Age, Years | Kruskal–Wallis Test | ||||
|---|---|---|---|---|---|---|
| 1+ | 2+ | 3+ | 4+ | H | p | |
| Males | ||||||
| Mean total length ± SE, mm | 62.5 ± 1.9 | 91.0 ± 3.1 | 110.0 ± 5.0 | - | 22,558 | <0.001 |
| Mean body weight ± SE, g | 1.48 ± 0.13 | 4.90 ± 0.31 | 8.50 ± 1.00 | - | 22.504 | <0.001 |
| No. fish examined | 20 | 10 | 2 | 0 | ||
| Females | ||||||
| Mean total length ± SE, mm | 68.2 ± 2.0 | 89.5 ± 2.0 | 108.3 ± 1.4 | 124.2 ± 1.7 | 47.943 | <0.001 |
| Mean body weight ± SE, g | 1.73 ± 0.15 | 4.85 ± 0.40 | 9.42 ± 0.34 | 13.67 ± 1.33 | 48.106 | <0.001 |
| No. fish examined | 22 | 20 | 12 | 3 | ||
| Kruskal–Wallis Test | ||||||
| Total length | H = 3.029, p = 0.082 | H = 1.031, p = 0.310 | H = 1.655, p = 0.198 | - | ||
| Body weight | H = 2.135, p = 0.144 | H = 0.436, p = 0.509 | H = 0.300, p = 0.584 | - | ||
| Parameter | Individual Fecundity, Eggs | |||
|---|---|---|---|---|
| Mean ± SE | Variations | No. Fish Examined | ||
| Total length, mm | 81–90 | 148 ± 1 | 147–150 | 2 |
| 91–100 | 150 ± 24 | 100–193 | 4 | |
| 101–110 | 225 ± 19 | 145–316 | 11 | |
| 111–120 | 239 ± 29 | 148–268 | 4 | |
| 121–130 | 250 ± 75 | 162–307 | 2 | |
| Body weight, g | 4–6 | 125 ± 16 | 100–186 | 5 |
| 6–8 | 238 ± 31 | 193–307 | 4 | |
| 8–10 | 225 ± 23 | 145–316 | 8 | |
| 10–12 | 205 ± 30 | 148–268 | 5 | |
| >14 | 307 | - | 1 | |
| Age, years | 2+ | 186 ± 30 | 100–316 | 9 |
| 3+ | 217 ± 19 | 145–268 | 12 | |
| 4+ | 250 ± 75 | 162–307 | 2 | |
| Prey | FO, % | Total Length, mm | Total | ||
|---|---|---|---|---|---|
| 41–70 | 71–100 | >100 | |||
| Isopoda | 1.1 | - | 0.8 | - | 0.3 |
| Amphipoda | 49.4 | 54.9 | 34.1 | 7.3 | 22.1 |
| Mysidacea | 27.0 | 28.9 | 19.6 | 48.0 | 35.4 |
| Decapoda | 1.1 | 4.5 | - | - | 0.4 |
| Teleostei, including: | 18.0 | 11.7 | 45.5 | 44.1 | 41.8 |
| Gymnocanthus tricuspis, juv. | 3.4 | - | 7.8 | 22.2 | 14.6 |
| Triglops pingelii, juv. | 1.1 | - | - | 7.0 | 3.6 |
| Other teleosts, juv. | 13.5 | 11.7 | 37.7 | 14.9 | 23.6 |
| Proportion of empty stomachs, % | 34.5 | 7.9 | 18.2 | 19.1 | |
| Index of stomach fullness, %oo | 156 | 177 | 147 | 163 | |
| No. fish examined | 29 | 38 | 22 | 89 | |
| Parameter | T. nybelini | T. murrayi | T. jordani | T. pingelii | T. forficatus | T. scepticus | |
|---|---|---|---|---|---|---|---|
| AO | PO | ||||||
| Maximum total length, mm | 170 | 200 | 200 | 180 | 232 | 323 | 354 |
| Maximum total length (males/females), mm | 125/170 | 108/170 | 170/200 | 120/150 | 200/220 | 250/300 | 250/260 |
| Maximum body weight (males/females), g | No data | No data | No data | 9/15 | 23/50 | 66/111 | 136/138 |
| Length–weight relationship | No data | No data | W = 0.0389TL2.3317 | W = 0.05496TL3.542 | W = 0.0110TL2.8675 | No data | W = 0.0025TL3.4871 |
| Maximum age, years | 7 | 10 | No data | 9 | 6 | 7 | 9 |
| Maximum age (males/females), years | No data | No data | No data | No data | 5/6 | 5/6 | 8/8 |
| Individual fecundity, eggs * | No data 300–600 | No data 100–2739 | No data No data | 215 100–730 | 1800 No data | 1700 No data | 3100 No data |
| Feeding type | Plankto- benthophage | Bentho- planktophage | Necto- benthophage | Necto-bentho- ichthyophage | Necto- benthophage | Bentho- macro- planktophage | Bentho-macro- planktophage |
| Habitation depths, m ** | 10–930 117–600 | 7–500 50–355 | 15–460 38–256 | 5–431 100–200 | 5–745 10–355 | 20–470 75–200 | 25–925 100–380 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokranov, A.M.; Emelin, P.O.; Orlov, A.M. Small and Abundant but Understudied Ribbed Sculpin Triglops pingelii (Cottidae, Teleostei) from the Kara Sea (Siberian Arctic): Distribution, Biology, and Comparison with Congeners. Diversity 2022, 14, 853. https://doi.org/10.3390/d14100853
Tokranov AM, Emelin PO, Orlov AM. Small and Abundant but Understudied Ribbed Sculpin Triglops pingelii (Cottidae, Teleostei) from the Kara Sea (Siberian Arctic): Distribution, Biology, and Comparison with Congeners. Diversity. 2022; 14(10):853. https://doi.org/10.3390/d14100853
Chicago/Turabian StyleTokranov, Alexey M., Pavel O. Emelin, and Alexei M. Orlov. 2022. "Small and Abundant but Understudied Ribbed Sculpin Triglops pingelii (Cottidae, Teleostei) from the Kara Sea (Siberian Arctic): Distribution, Biology, and Comparison with Congeners" Diversity 14, no. 10: 853. https://doi.org/10.3390/d14100853