New Molecular-Based Phylogeny of Mussel-Associated Mites Reveals a New Subgenus and Three New Species Representing an Example of a Host-Driven Radiation in Indochina and Confirms the Concept of Division of the Genus Unionicola Haldeman, 1842 (Acari: Unionicolidae) into Numerous Subgenera
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collecting of Specimens, Map Creation, and Morphological Research
2.2. DNA Analyses
2.3. Phylogenetic Analyses
2.4. Nomenclatural Acts
3. Results
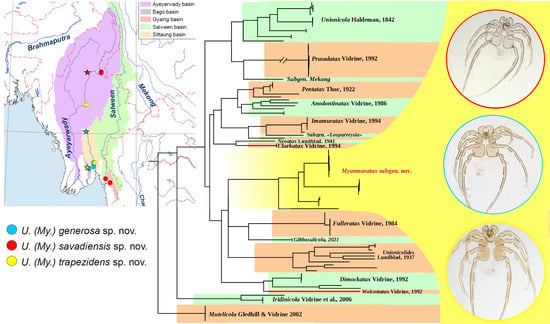
3.1. New Subgenus of Mussel-Associated Mites from Southeast Asia
3.2. Taxonomic Account
- Family Unionicolidae Oudemans, 1909
- Genus Unionicola Haldeman, 1842
- Subgenus Myanmaratax Chapurina, Vidrine, Kondakov, Vikhrev and Bolotov subgen. nov.
- LSID: urn:lsid:zoobank.org:act:C242AA35-C194-40E1-B3EA-4D0A924B6F09 (accessed on 27 September 2022)
- Type species. Unionicola (Myanmaratax) savadiensis subgen. and sp. nov.
- Unionicola (Myanmaratax) savadiensis Chapurina, Vidrine, Kondakov, Vikhrev and Bolotov subgen. and sp. nov.




- Host range. This species is a narrow host specialist, which is known to occur on the gills of two Lamellidens species, namely L. savadiensis and L. generosus (Figure 3C,D).
- Unionicola (Myanmaratax) trapezidens Chapurina, Vidrine, Kondakov, Vikhrev and Bolotov sp. nov.

3.3. Morphometric Analysis
- For U. (My.) savadiensis, females are significantly larger than males by the idiosoma length (LI) and by other tested parameters (Wilk’s Lambda = 0.00033; F1,9 = 330, 14; p = 0.043).
- For U. (My.) generosa, the lengths of PCG, P-2, P-3, I-L-1 between males and females were similar and do not show statistically significant differences between sexes by the length of tested morphological structures (Wilk’s Lambda = 0.001; F1,9 = 216.89; p = 0.053).
- For U. (My.) trapezidens, the lengths of only two of the 22 tested structures (P-2 and P-3) were similar for males and females, while morphological differences between sexes were not significant (Wilk’s Lambda = 0.008; F1,5 = 29.97; p = 0.136).
| Dependent Variable | Mixed Sample (Both Sexes) | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Square Sum, SS | F2,22 | p | Square Sum, SS | F2,22 | p | Square Sum, SS | F2,22 | p | |
| LI | 169,512.94 | 6.53 | 0.006 | 64,837.75 | 11.53 | 0.002 | 154,223.66 | 3.33 | 0.074 |
| wGF | 712.24 | 2.41 | 0.113 | 344.36 | 1.21 | 0.335 | 1867.61 | 6.11 | 0.016 |
| PCG | 569.08 | 0.67 | 0.522 | 591.84 | 0.53 | 0.602 | 915.45 | 1.56 | 0.253 |
| P-1 | 158.44 | 8.71 | 0.002 | 117.40 | 7.41 | 0.009 | 54.73 | 2.67 | 0.114 |
| P-2 | 1625.47 | 4.23 | 0.028 | 1153.36 | 3.24 | 0.078 | 541.00 | 1.31 | 0.309 |
| P-3 | 345.03 | 1.23 | 0.313 | 92.21 | 0.24 | 0.795 | 370.77 | 2.18 | 0.159 |
| P-4 | 2577.12 | 18.34 | 0.000 | 1294.61 | 5.65 | 0.020 | 1443.49 | 27.77 | 0.000 |
| P-5 | 51.99 | 0.80 | 0.463 | 116.60 | 1.85 | 0.203 | 11.84 | 0.18 | 0.841 |
| I-L-1 | 203.78 | 1.96 | 0.165 | 90.95 | 0.87 | 0.448 | 134.48 | 1.30 | 0.311 |
| I-L-2 | 578.65 | 3.56 | 0.046 | 336.36 | 1.12 | 0.361 | 558.10 | 22.59 | 0.000 |
| I-L-3 | 2694.11 | 16.41 | 0.000 | 1718.73 | 5.46 | 0.023 | 1462.02 | 108.04 | 0.000 |
| I-L-4 | 2521.37 | 5.80 | 0.009 | 2209.81 | 2.81 | 0.103 | 2067.17 | 24.97 | 0.000 |
| I-L-5 | 6515.00 | 14.49 | 0.000 | 3742.71 | 4.58 | 0.036 | 3952.99 | 47.70 | 0.000 |
| I-L-6 | 3071.93 | 56.74 | 0.000 | 1515.34 | 18.99 | 0.000 | 1621.22 | 56.90 | 0.000 |
| IV-L-1 | 876.21 | 3.61 | 0.044 | 193.14 | 0.62 | 0.556 | 862.48 | 4.98 | 0.029 |
| IV-L-2 | 183.82 | 0.79 | 0.468 | 576.20 | 1.75 | 0.220 | 83.46 | 0.61 | 0.563 |
| IV-L-3 | 760.33 | 0.87 | 0.434 | 2058.31 | 1.40 | 0.287 | 1281.20 | 4.50 | 0.037 |
| IV-L-4 | 6942.79 | 3.93 | 0.035 | 3079.19 | 0.98 | 0.406 | 10,032.55 | 25.73 | 0.000 |
| IV-L-5 | 23,001.09 | 9.60 | 0.001 | 6875.27 | 1.77 | 0.215 | 23,021.99 | 25.17 | 0.000 |
| IV-L-6 | 5564.89 | 27.75 | 0.000 | 2231.11 | 7.47 | 0.009 | 3754.15 | 36.71 | 0.000 |
| III-L-5 | 1228.85 | 1.00 | 0.384 | 3241.41 | 1.55 | 0.256 | 5401.17 | 15.01 | 0.001 |
| III-L-6 | 1283.30 | 6.45 | 0.006 | 767.77 | 3.43 | 0.070 | 1388.56 | 7.97 | 0.007 |
| total | Wilk’s Lambda = 0.000003 F2,22 = 25.709; p = 0.038 | Wilk’s Lambda = 0.0001 F2,22 = 8994; p = 0.104 | Wilk’s Lambda = 0.0002 F2,22 = 6.796; p = 0.135 | ||||||
- The PCA scatterplot by the length of 22 morphological structures on the first 2 canonical axes showed that the males of the three species overlap with each other (Figure 8).
- In particular, the samples of U. (My.) generosa and U. (My.) trapezidens males share similar ordination in the length space by both axes, and only the sample of U. (My.) savadiensis has a distinct position from the two other species. In contrast, the sample of each species’ females represents a separate cloud in the morphometric space (Figure 8).
- The pairwise comparisons indicated that the lengths of three morphological structures could be used for species identification (Table 4).
- The idiosoma length (LI) of U. (My.) savadiensis is significantly smaller compared with that of U. (My.) generosa and U. (My.) trapezidens (Table 4).
- The tarsus and tibia lengths of the first and forth walking legs (IL-6 and IV-L-5, respectively) of U. (My.) trapezidens are significantly larger than those for the two other species, namely U. (My.) generosa and U. (My.) savadiensis (Table 4).
- Only male examples of U. (My.) generosa do not share significant differences in the length of idiosoma and leg segments compared with those in the two other species, indicating the need for additional morphological features for species identification.
- As for females, the pairwise comparisons have shown that the linear dimensions of telofemur, genu, and tibia of the first and fourth walking legs (I-L-3, I-L-4, and IV-L-5, respectively) were significantly different between the three new species (Table 4). The longest leg segments were detected for U. (My.) trapezidens, while U. (My.) generosa revealed the shortest ones (Table 4).
- Thus, the ratios of linear dimensions of leg segments and idiosoma are useful for identification of the studied cryptic mite species in the subgenus Myanmaratax.
3.4. Taxonomic Key
| 1 | P-2 with 3 lateral setae and P-3 with 1 seta. Distal seta dorsal to claws at the end of each walking leg is prominent……… | U. (My.) brandti Vidrine, 1985 |
| – | P-2 with 4 lateral setae and P-3 without setae. Distal seta dorsal to claws at the end of each walking leg is insignificantly noticeable……… | 2 |
| 2 | I-L-6 length is 141–144 µm (average is 143 µm); IV-L-6 is 319–338 (average is 330). I-L-4 with a row of lateral three awl-shaped prominent setae and two thin setae. The ratio of the width of the genital field to its length is 1.35 (varies from 1.28 to 1.44)……… | U. (My.) trapezidens sp. nov. |
| – | Average I-L-6 length is 115–120 µm (the size varies from 108 to 132 µm). I-L-4 with a row of lateral setae of the same shape (awl-shaped and blunt). The ratio of the width of the genital field to its length is 1.17–1.18 (varies from 0.95 to 1.34)……… | 3 |
| 3 | The idiosoma size is 745–827 (average is 793). The ratio of the whole length of the body to the length of posterior coxal group for males is 2.7……… | U. (My.) savadiensis sp. nov. |
| – | Length of the idiosoma 860–1054 (average is 945). The ratio of the whole length of the body to the length of posterior coxal group for males is 3.1……… | U. (My.) generosa sp. nov. |
| 1 | IV-L-3 is 200–300 µm, IV-L-4 is less than 430 µm, IV-L-5 varies from 310 to 525, IV-L-6 is 250–300 µm……… | U. (My.) brandti Vidrine, 1985 |
| – | IV-L-3 is more than 320 µm, IV-L-4 is more than 437 µm, IV-L-5 is more than 563 µm, IV-L-6 is more than 330 µm……… | 2 |
| 2 | The length of I-L-4 varies from 300 to 304 µm; I-L-5 is 317–329 µm, I-L-6 is 158–164 µm, I-L-2 with one/one or one/two ventrodistal swimming setae (both variants of the trait may be observed within the same specimen)……… | U. (My.) trapezidens sp. nov. |
| – | The length of I-L-4 less than 292 µm, length of I-L-5 less than 302 µm, I-L-6 less than 150 µm, I-L-2 with two or more ventrodistal swimming setae……… | 3 |
| 3 | The shape of II-L-6 and III-L-6 is a dumbbell with a well-defined expansion closer to the basis of the tarsus and a narrowing located distally……… | U. (My.) generosa sp. nov. |
| – | The shape of II-L-6 is weakly dumbbell, and the shape of III-L-6 is a dumbbell with a narrowing located at the distal part of the tarsus……… | U. (My.) savadiensis sp. nov. |
4. Discussion
4.1. Fauna of Mussel-Associated Unionicola Mites in Southeast and South Asia
4.2. Subgeneric Taxonomic Concept of the Genus Unionicola
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitchell, R. Host exploitation of two closely related water mites. Evolution 1967, 21, 59–75. [Google Scholar] [CrossRef] [Green Version]
- Böttger, K. Parasitologische Beziehungen zwischen Wassermilben und Trichopteren. Zool. Anz. 1972, 188, 154–156. [Google Scholar]
- Jones, R.K.H. Parasitism by Unionicola spp. larvae on chironomids. Hydrobiologia 1978, 60, 81–87. [Google Scholar] [CrossRef]
- Baker, R.A. Development and life strategies in mussel mites. In The Acari: Reproduction, Development and Life History Strategies; Schuster, R., Murphy, P.W., Eds.; Chapman and Hall: London, UK, 1991; pp. 65–73. [Google Scholar]
- Edwards, D.D.; Dimock Jr, R.V. Life history characteristics of larval Unionicola (Acari: Unionicolidae) parasitic on Chironomus tentans (Diptera: Chironomidae). J. Nat. Hist. 1995, 29, 1197–1208. [Google Scholar] [CrossRef]
- Proctor, H.C.; Smith, I.M.; Cook, D.R.; Smith, B.P. Subphylum Chelicerata, Class Arachnida. In Ecology and General Biology: Thorp and Covich’s Freshwater Invertebrates; Thorp, J., Rogers, D.C., Eds.; Academic Press: New York, NY, USA; London, UK, 2015; pp. 599–660. [Google Scholar]
- Chapurina, Y.E.; Bolotov, I.N.; Vidrine, M.F.; Vikhrev, I.V.; Lunn, Z.; Chan, N.; Win, T.; Bespalaya, Y.V.; Aksenova, O.V.; Gofarov, M.Y.; et al. Taxonomic richness and host range of tropical Asian mussel-associated mite assemblage (Acari: Unionicolidae) with description of a new subgenus and species of parasitic mite from freshwater pearl mussels (Unionida: Margaritiferidae). J. Zool. Syst. Evol. Res. 2021, 59, 613–634. [Google Scholar] [CrossRef]
- Wen, C.; Zhu, Z. Seven species of water mites in the genus Unionicola from Jiangxi (Acari: Unionicolidae). Acta Zootaxon Sin. 1999, 24, 30–37. [Google Scholar]
- Edwards, D.D.; Vidrine, M.F. Mites of Freshwater Mollusks; Malcolm, F., Ed.; Vidrine: Eunice, LA, USA, 2013; 336p. [Google Scholar]
- Savatenalinton, S.; Smit, H. New records of water mites from standing waters in Thailand, with the description of nine new species (Acari: Hydrachnidia). Zootaxa 2017, 4312, 69–91. [Google Scholar] [CrossRef]
- Lewisch, E.; Arnold, F.; Fuehrer, H.-P.; Harl, J.; Reichart, U.; Handschuh, S.; Thielen, F.; El-Matbouli, M. First description of freshwater mite Unionicola sauerensis sp. nov. infesting thick-shelled river mussel Unio crassus. Dis. Aquat. Organ. 2021, 145, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Smit, H. The water mites of Western Australia (Acari: Hydrachnidia), with the description of 13 new species. Acarologia 2021, 61, 928–966. [Google Scholar] [CrossRef]
- Ding, Z.; Jin, D.; Guo, J.; Yi, T. Three new species of the subgenus Unionicola Haldeman, 1842 (Acari, Hydrachnidia, Unionicolidae) from Guizhou, China. Zootaxa 2019, 4658, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Chapurina, Y.E.; Kondakov, A.V.; Chan, N.; Vikhrev, I.V.; Bolotov, I.N.; Konopleva, E.S.; Win, T.; Lunn, Z. A new species Unionicola (Dimockatax stat. rev.) haungthayawensis sp. nov.(Trombidiformes: Unionicolidae) from the freshwater mussel Lamellidens generosus (Gould, 1847) in Myanmar. Ecol. Monten. 2022, 56, 28–39. [Google Scholar] [CrossRef]
- Bolotov, I.N.; Kondakov, A.V.; Vikhrev, I.V.; Aksenova, O.V.; Bespalaya, Y.V.; Gofarov, M.Y.; Kolosova, Y.S.; Konopleva, E.S.; Spitsyn, V.M.; Tanmuangpak, K.; et al. Ancient River Inference Explains Exceptional Oriental Freshwater Mussel Radiations. Sci. Rep. 2017, 7, 2135. [Google Scholar] [CrossRef] [PubMed]
- Bolotov, I.N.; Konopleva, E.S.; Vikhrev, I.V.; Gofarov, M.Y.; Lopes-Lima, M.; Bogan, A.E.; Lunn, Z.; Chan, N.; Win, T.; Aksenova, O.V.; et al. New freshwater mussel taxa discoveries clarify biogeographic division of Southeast Asia. Sci. Rep. 2020, 10, 6616. [Google Scholar] [CrossRef] [Green Version]
- Bolotov, I.N.; Klass, A.L.; Kondakov, A.V.; Vikhrev, I.V.; Bespalaya, Y.V.; Gofarov, M.Y.; Filippov, B.Y.; Bogan, A.E.; Lopes-Lima, M.; Lunn, Z.; et al. Freshwater mussels house a diverse mussel-associated leech assemblage. Sci. Rep. 2019, 9, 16449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolotov, I.N.; Vikhrev, I.V.; Kondakov, A.V.; Konopleva, E.S.; Gofarov, M.Y.; Aksenova, O.V.; Tumpeesuwan, S. New taxa of freshwater mussels (Unionidae) from a species-rich but overlooked evolutionary hotspot in Southeast Asia. Sci. Rep. 2017, 7, 11573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolotov, I.N.; Pfeiffer, J.M.; Konopleva, E.S.; Vikhrev, I.V.; Kondakov, A.V.; Aksenova, O.V.; Gofarov, M.Y.; Tumpeesuwan, S.; Win, T. A new genus and tribe of freshwater mussel (Unionidae) from Southeast Asia. Sci. Rep. 2018, 8, 10030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolotov, I.N.; Konopleva, E.S.; Vikhrev, I.V.; Lopes-Lima, M.; Bogan, A.E.; Lunn, Z.; Chan, N.; Win, T.; Aksenova, O.V.; Gofarov, M.Y.; et al. Eight new freshwater mussels (Unionidae) from tropical Asia. Sci. Rep. 2019, 9, 12053. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, J.M.; Graf, D.L.; Cummings, K.S.; Page, L.M. Taxonomic revision of a radiation of South-East Asian freshwater mussels (Unionidae: Gonideinae: Contradentini+ Rectidentini). Invertebr. Syst. 2021, 35, 394–470. [Google Scholar] [CrossRef]
- Edwards, D.D.; Vidrine, M.F. Host specificity among Unionicola spp. (Acari: Unionicolidae) parasitizing freshwater mussels. J. Parasitol. 2006, 92, 977–983. [Google Scholar] [CrossRef]
- Edwards, D.D.; Vidrine, M.F. Host Diversity Affects Parasite Diversity: A Case Study Involving Unionicola spp. Inhabiting Freshwater Mussels. J. Parasitol. 2020, 106, 675–678. [Google Scholar] [CrossRef]
- Chapurina, Y.E.; Vikhrev, I.V.; Kondakov, A.V.; Tanmuangpak, K. A new Najadicola species (Acari: Hydrachnidia: Pionidae) from Asia. Ecol. Monten. 2019, 24, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Bogan, A.E.; Hoeh, W.R. On becoming cemented: Evolutionary relationships among the genera in the freshwater bivalve family Etheriidae (Bivalvia: Unionoida). Geol. Soc. Spec. Publ. 2000, 177, 159–168. [Google Scholar] [CrossRef]
- Graf, D.L.; Cummings, K.S. A ‘big data’ approach to global freshwater mussel diversity (Bivalvia: Unionoida), with an updated checklist of genera and species. J. Molluscan. Stud. 2021, 87, eyaa034. [Google Scholar] [CrossRef]
- Majumder, M.Z.R.; Pal, S.G. Adaptations of Unionicola sp., a freshwater mite on Lamellidens marginalis from Bengal. Bicovas 1988, 1, 191–202. [Google Scholar]
- Vidrine, M.F. Majumderatax, new subgenus (Acari: Unionicolidae: Unionicolinae: Unionicola), in Europe and Asia. Int. J. Acarol. 1993, 19, 101–102. [Google Scholar] [CrossRef]
- Vidrine, M.F. North American Najadicola and Unionicola: Systematics and coevolution; Gail, Q., Ed.; Vidrine Collectibles: Eunice, LA, USA, 1996; p. 146. [Google Scholar]
- Wu, H.B.; Wen, C.G.; Guo, W. Sequence variation of the mitochondrial 12S rRNA gene among Unionicola (Wolcottatax) arcuata (Acari: Unionicolidae) from freshwater mussels in China. Int. J. Acarol. 2012, 38, 394–401. [Google Scholar] [CrossRef]
- Ernsting, B.R.; Edwards, D.D.; Timbrook, T.A.; Frerichs, M.M. Preliminary evidence of cryptic species among host-associated populations of Unionicola hoesei (Acari: Unionicolidae). Int. J. Acarol. 2014, 40, 358–365. [Google Scholar] [CrossRef]
- Stålstedt, J.; Bergsten, J.; Ronquist, F. “Forms” of water mites (Acari: Hydrachnidia): Intraspecific variation or valid species? Ecol. Evol. 2013, 3, 3415–3435. [Google Scholar] [CrossRef]
- Lehner, B.; Grill, G. Global river hydrography and network routing: Baseline data and new approaches to study the world’s large river systems. Hydrol. Process. 2013, 27, 2171–2186. [Google Scholar] [CrossRef]
- Lehner, B.; Verdin, K.; Jarvis, A. New global hydrography derived from spaceborne elevation data. Eos Trans. AGU 2008, 89, 93–94. [Google Scholar] [CrossRef]
- Vidrine, M.F. Fulleratax, new subgenus (Acari: Unionicolidae: Unionicolinae: Unionicola), in Southeast Asia. Int. J. Acarol. 1984, 10, 229–233. [Google Scholar] [CrossRef]
- Vidrine, M.F. Three new species of Unionicola (Acari Unionicolidae: Unionicolinae) inhabiting fresh water mussels (Unionacea) in Southeast Asia. Int. J. Acarol. 1985, 11, 125–131. [Google Scholar] [CrossRef]
- Vidrine, M.F. Five new species in the subgenus Parasitatax (Acari: Unionicolidae: Unionicola) from North America and Asia, with a re-evaluation of related species. Int. J. Acarol. 1986, 12, 141–153. [Google Scholar] [CrossRef]
- Hammer, Ø. PAST-Paleontological Statistics Reference Manual, v. 3.05.; Natural History Museum, University of Oslo: Oslo, Norway, 2015. [Google Scholar]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989; p. 1659. [Google Scholar]
- Lobo, J.; Costa, P.M.; Teixeira, M.A.L.; Ferreira, M.S.G.; Costa, M.H.; Costa, F.O. Enhanced primers for amplification of DNA barcodes from a broad range of marine metazoans. BMC Ecol. 2013, 13, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Huelsenbeck, J.P. MrBayes.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES science gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010. [Google Scholar]
- Viets, K.H. Neue Wassermilben (Hydrachnellae, Acari) von Borneo, Indonesia. Abh. Hrsg. Vom Nat. Ver. Zu Brem. 1957, 35, 8–23. [Google Scholar]
- Viets, K.H. Indische Wassermilben. Zool. Jahrb. Abt. Syst. Geogr. Biol. Tiere 1926, 52, 369–394. [Google Scholar]
- Hevers, J. Unionicola species (Acari: Hydrachnidia: Unionicolidae) from Madagascar. Stuttg. Beiträge Zur Nat. A 2012, 5, 49–71. [Google Scholar]
- Pešić, V.; Zawal, A. A new species in the water mite subgenus Majumderatax Vidrine, 1993 from Sri Lanka (Acari: Hydrachnidia). Zootaxa 2018, 4457, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Smit, H. Water Mites of the World with Keys to the Families, Subfamilies, Genera and Subgenera (Acari: Hydrachnidia); Nederlandse Entomologische Vereniging: Leiden, The Netherlands, 2020; p. 774. [Google Scholar]
- Williams, P.H.; Cameron, S.A.; Hines, H.M.; Cederberg, B.; Rasmont, P. A simplified subgeneric classification of the bumblebees (genus Bombus). Apidologie 2008, 39, 46–74. [Google Scholar] [CrossRef] [Green Version]
- Harvey, M.S. The Australian Water Mites: A Guide to the Families and Genera. Monographs in Invertebrate Taxonomy; CSIRO Publishing: Collingwood, VIC, Australia, 1998; Volume 4, p. 150. [Google Scholar]
- Ernsting, B.R.; Edwards, D.D.; Vidrine, M.F.; Myers, K.S.; Harmon, C.M. Phylogenetic relationships among species of the subgenus Parasitatax (Acari: Unionicolidae: Unionicola) based on DNA sequence of the mitochondrial cytochrome oxidase I gene. Int. J. Acarol. 2006, 32, 195–202. [Google Scholar] [CrossRef]
- Edwards, D.D.; Vidrine, M.F.; Ernsting, B.R. Phylogenetic relationships among Unionicola (Acari: Unionicolidae) mussel-mites of North America based on mitochondrial cytochrome oxidase I sequences. Zootaxa 2010, 2537, 47–57. [Google Scholar] [CrossRef]



| Subgenus | Species | Type Locality | Distribution | Known Hosts * | Localization | Reference |
|---|---|---|---|---|---|---|
| Dimockatax Vidrine 1992 | U. (Di.) tumidoides Vidrine, 1986 | Lam Seio Yai, Ampoe Suwannaphum, Roi Et Province, Thailand | Mekong Basin, Thailand | Ensidens ingallsianus (Lea, 1852) [type host] | Gills | [37] |
| U. (Di.) haungthayawensis Chapurina et al., 2022 | Haungthayaw River, upstream of Kawkareik town, 16°28′17″ N, 98°13′5″ E, Salween River basin, Myanmar | Haungthayaw River basin; Winyaw River, Ataran River basin, Myanmar. | Lamellidens generosus (Gould, 1847) [type host]; | Gills | [14], this study | |
| Fulleratax Vidrine, 1984 | U. (Fu.) davisi Vidrine, 1984 | Huai Phen, Amphoe Phen, Udon Thani Province, Thailand | Mekong Basin, Thailand | Pilsbryoconcha compressa (Martens, 1860) [type host] | Gills | [35] |
| U. (Fu.) robacki Vidrine, 1984 | Lam Khlong, Boribun, Ban Choho, Nakhon Ratchasima, Nakhon Ratchasima Province, Thailand | Mekong Basin in Thailand and Laos | Hyriopsis khoratensis Pfeiffer et al., 2021 [type host] | Gills | [35], this study | |
| Gibbosulicola Chapurina et al., 2021 | U. (Gi.) sella Chapurina, Bolotov, Vidrine, Kondakov & Vikhrev, 2021 | Thauk Ye Kupt River, 19°18′27″ N, 96°43′18″ E, Sittaung Basin, Myanmar | Not known beyond its type locality in Myanmar | Gibbosula laosensis ssp. woodthorpi (Godwin-Austen, 1919) [type host] | Gills | [7] |
| Imamuratax Vidrine 1994 | U. (Im.) heardi Vidrine, 1985 | Mekong River, Ban Dan, island site no. 4, Ubon Province, Thailand | Mekong and Mae Klong rivers, Thailand | Hyriopsis khoratensis Pfeiffer et al., 2021 [type host]; H. myersiana (Lea, 1856) | Mantle | [36] |
| U. (Im.) neokoenikei Viets, 1957 | The upper course of the river Kapuas (Kalimantan-Borneo) | Kapuas Basin, western Borneo | Hyriopsis velthuizeni (Schepman, 1896) [type host] | ** Mantle | [47] | |
| U. (Im.) scutigera Viets, 1926 | Bhandardaha Beel, Murshidabad District, Bengal (from freshwater mussels Lamellidens spp.) | India | Lamellidens sp. [type host] | Mantle | [48] | |
| Myanmaratax subgen. nov. | U. (My.) savadiensis subgen. and sp. nov. = Subgen. sp.B1 Chapurina et al., 2021 | Indaw lake, Thett Kel Chin village, 24°15′59″ N 96°7′22″ E, Ayeyarwady basin, Myanmar | Ayeyarwady, Salween, and Haungthayaw basins, Myanmar | Lamellidens savadiensis (Nevill, 1877) [type host]; L. generosus (Gould, 1847) | Gills | This study |
| U. (My.) generosa sp. nov. = Subgen. sp.B2 Chapurina et al., 2021 | Thay Dam, 20°9′14″ N 96°6′53″ E, Sittaung basin, Myanmar | Bago and Sittaung basins, Myanmar | Lamellidens savadiensis (Nevill, 1877) [type host]; L. generosus (Gould, 1847) | Gills | This study | |
| U. (My.) trapezidens sp. nov. = Subgen. sp.B3 Chapurina et al., 2021 | Kyauk Phar Stream, 17°39′57″ N 96°14′47″ E, Bago basin, Myanmar | Ayeyarwady, Bago, and Sittaung basins, Myanmar | Trapezidens angustior (Hanley & Theobald, 1876) [type host]; T. dolichorhynchus (Tapparone-Canefri, 1889) | Gills | This study | |
| U. (My.) brandti Vidrine, 1985 comb. nov. | Khlong Chonprathan, Ban Chonprathan, Phet Buri Province, Thailand | Thailand and Laos | Lens contradens (Lea, 1838) [type host]; L. eximius (Lea, 1856); L. misellus (Morelet, 1865); Ensidens ingallsianus (Lea, 1852); Thaiconcha callifera (Martens, 1860) [doubtful] | Gills | [36], this study | |
| Pentatax Thor, 1923 | U. (Pe.) thaiensis Vidrine, 1985 | Maenam Mun, Ban Bao Yai, Burinam Province, Thailand | Mun River, Mekong Basin, Thailand | Thaiconcha callifera (Martens, 1860) [type host]; Monodontina cambodjensis (Petit, 1865); Pilsbryoconcha compressa (Martens, 1860); P. exilis (Lea, 1838) | Mantle | [36] |
| Prasadatax Vidrine, 1992 | U. (Pr.) diversipes Viets, 1926 | Bhandardaha Beel, Murshidabad District, Bengal (from freshwater mussels Lamellidens spp.); edge of Inle Lake, at Fort Stedman, Yawnghwe State, South Shan States, Burma (free-living) | Ganges Basin in India, and Ayeyarwady and Salween basins in Myanmar | Lamellidens sp. [type host] | Gills | [48], this study |
| Unionicola Haldeman, 1842 | U. (Un.) thienemanni Viets, 1957 | The upper course of the river Kapuas (Kalimantan-Borneo) | Kapuas Basin, western Borneo, and [probably] Thailand | Hyriopsis velthuizeni (Schepman, 1896) [type host]; H. myersiana (Lea, 1856); H. khoratensis Pfeiffer et al., 2021; Ensidens ingallsianus (Lea, 1852); Thaiconcha callifera (Martens, 1860) [doubtful] | ** Gills | [37,47] |
| Species | Mean COI p-Distance from the Nearest Neighbor, % | The Nearest Neighbor of a Species | Fixed Nucleotide Differences Based on the Sequence Alignment of Congeners | |
|---|---|---|---|---|
| COI | 28S rRNA | |||
| U. (My.) savadiensis sp. nov. | 14.5 | U. sella | 389 T, 443 A, 479 A, 503 G, 521 G, 599 T, 601 C | 332 A, 350 G, 421 A, 514 C |
| U. (My.) generosa sp. nov. | 15.1 | U. sella | 92 C, 205 A, 279 A, 335 G, 356 G, 374 G, 431 C, 479 C | 211 C, 234 A, 341 C, 423 G, 440 G, 466 T, 495 C, 496 A, 513 C |
| U. (My.) trapezidens sp. nov. | 16.4 | U. agilex Wen, Hu and Zhu, 2008 | 83 C, 95 C, 164 C, 170 A, 173 C, 184 T, 190 A, 209 G, 248 G, 269 A, 284 T, 305 G, 332 A, 371 A, 590 C, 602 G | 187 C, 188 G, 237 G, 350 T, 429 C, 432 G, 438 C, 479 T, 482 A |
| Morphologic Parameter | Males (M ± SE *) | Females (M ± SE) | ||||
|---|---|---|---|---|---|---|
| U. (My.) savadiensis | U. (My.) generosa | U. (My.) trapezidens | U. (My.) savadiensis | U. (My.) generosa | U. (My.) trapezidens | |
| LI | 792.51 ± 13.58a ** | 945.14 ± 29.68b | 899.33 ± 11.66b | - | - | - |
| P-4 | 109.52 ± 4.84a | 118.99 ± 4.79ab | 135.79 ± 4.09b | 147.72 ± 1.50a | 145.013 ± 1.11a | 171.06 ± 5.68b |
| I-L-3 | 144.65 ± 3.74a | 158.93 ± 6.71ab | 174.64 ± 4.09b | 200.74 ± 0.66a | 194.37 ± 1.37b | 221.79 ± 2.00c |
| I-L-4 | - | - | - | 281.27 ± 2.65a | 268.54 ± 3.45b | 301.74 ± 0.98c |
| I-L-5 | 203.95 ± 5.72a | 224.29 ± 9.94ab | 248.35 ± 12.08b | 288.19 ± 3.06a | 276.72 ± 2.20a | 322.03 ± 3.59b |
| I-L-6 | 115.80 ± 2.53a | 119.49 ± 3.20a | 142.86 ± 0.84b | 138.90 ± 1.90a | 133.73 ± 1.05a | 162.18 ± 2.08b |
| IV-L-5 | 301.95 ± 5.56a | 297.66 ± 5.30a | 330.03 ± 5.55b | 363.20 ± 1.36a | 338.96 ± 3.43b | 382.40 ± 6.73c |
| III-L-6 | 203.99 ± 4.85a | 212.02 ± 4.20ab | 224.22 ± 6.20b | 248.98 ± 2.42a | 230.57 ± 6.11b | 254.64 ± 2.46a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chapurina, Y.E.; Konopleva, E.S.; Vidrine, M.F.; Vikhrev, I.V.; Lunn, Z.; Chan, N.; Win, T.; Kondakov, A.V.; Zubrii, N.A.; Bespalaya, Y.V.; et al. New Molecular-Based Phylogeny of Mussel-Associated Mites Reveals a New Subgenus and Three New Species Representing an Example of a Host-Driven Radiation in Indochina and Confirms the Concept of Division of the Genus Unionicola Haldeman, 1842 (Acari: Unionicolidae) into Numerous Subgenera. Diversity 2022, 14, 848. https://doi.org/10.3390/d14100848
Chapurina YE, Konopleva ES, Vidrine MF, Vikhrev IV, Lunn Z, Chan N, Win T, Kondakov AV, Zubrii NA, Bespalaya YV, et al. New Molecular-Based Phylogeny of Mussel-Associated Mites Reveals a New Subgenus and Three New Species Representing an Example of a Host-Driven Radiation in Indochina and Confirms the Concept of Division of the Genus Unionicola Haldeman, 1842 (Acari: Unionicolidae) into Numerous Subgenera. Diversity. 2022; 14(10):848. https://doi.org/10.3390/d14100848
Chicago/Turabian StyleChapurina, Yulia E., Ekaterina S. Konopleva, Malcolm F. Vidrine, Ilya V. Vikhrev, Zau Lunn, Nyein Chan, Than Win, Alexander V. Kondakov, Natalia A. Zubrii, Yulia V. Bespalaya, and et al. 2022. "New Molecular-Based Phylogeny of Mussel-Associated Mites Reveals a New Subgenus and Three New Species Representing an Example of a Host-Driven Radiation in Indochina and Confirms the Concept of Division of the Genus Unionicola Haldeman, 1842 (Acari: Unionicolidae) into Numerous Subgenera" Diversity 14, no. 10: 848. https://doi.org/10.3390/d14100848