4.1. Diet Composition of Lithodes santolla
The stomach content data analyzed in the present study characterized Nassau Bay southern king crabs as omnivorous feeders that consume a great diversity of prey including crustaceans, hydrozoans, other small benthic invertebrates (e.g., bivalves and bryozoans), algae, among other prey. These findings corroborate previous studies of populations located in different regions and lead us to conclude that
L. santolla has a generalist feeding strategy and the ability to exploit a wide variety of natural resources from coastal benthic waters [
2,
3,
4,
14,
15,
19,
62,
63]. Therefore,
L. santolla’s foraging strategy should significantly influence trophic dynamics, as strategies that incorporate a more varied prey favor more complex energetic pathways in the local benthic food web [
64].
Interestingly, studies of the diet composition of
L. santolla based on stomach content analyses are mostly restricted to juveniles or adults, but they can shift their diets. For example, Comoglio and Amin [
15] indicated that juveniles, between 40–70 mm CL from the Beagle Channel, consume mainly gastropod mollusks, crustaceans, bryozoans, and algae, finding a total of 20 different items. In early juveniles, that is, specimens between 8.40–49.04 mm CL from the Gulf of San Jorge, Argentina, Vinuesa et al. [
14] identified a total of 27 different food items, being red algae, ophiuroids, echinoderms, isopods, bivalve mollusks, polychaetes and bryozoans the most consumed prey. In adults between 70–119 mm CL also from the Gulf of San Jorge, Balzi [
42] recorded four main prey including fish, squat lobsters, echinoderms, and bivalve mollusks.
Our study shows significant differences in the feeding patterns of late juveniles and adults, whereby late juveniles consume more bivalves than any other group. Therefore, juveniles forage differently from adults, which is probably due to the availability of prey in the habitat which individuals use. Since southern king crabs’ sexual maturity varies in relation to region or depth [
5,
40,
65], care has been taken to assure the class sizes’ accuracy between late juveniles and adults reflected in the population under study.
Furthermore, southern king crabs are also known to have similar food preferences between males and females [
14,
15]. Conversely, our study detected differences in diet composition between sexes in both adults that were mostly related to prey item percentage. This result could indicate variations in prey availability in the foraging areas that could probably be attributable to differences in sexual behavior and ontogenetic patterns of habitat use [
66,
67]. Indeed, some evidence indicates that in the early spring season, females and males of
L. santolla migrate to shallow waters for molting and mating and then return to deeper waters in late spring [
40,
68]. Hence, foraging prey availability for
L. santolla of both sexes may match within their habitat during this reproductive migration.
L. santolla also appears to have an opportunistic scavenging strategy since the proportion of fish registered in diet composition among sexes and maturity stages presumably relies on the fish bait used in the crab traps (e.g., southern hake). Similar results were recorded in other fishing areas, where
L. santolla scavenges discarded fish, such as Argentine hake
Merluccius hubbsi, from trawlers [
42].
In general,
Lithodes santolla feed throughout the year, except for a few weeks during the molting period, when feeding ceases or decreases in intensity [
69]. This has been shown, for example, in
L. santolla in the Beagle Channel, where a high percentage of empty stomachs were recorded during the spring period [
15]. This may explain the results observed in our study, where although the number of entirely empty stomachs observed was minimal, a high percentage of stomachs were found only with traces of food.
On the other hand, the stomach content results provide evidence of intraspecific predation within the natural population of
L. santolla. The presence of crab carapace fragments inside the southern king crabs’ stomachs allows us to infer the possibility of cannibalistic behavior between juveniles and adults with unknown implications for this species’ growth, fitness, and reproductive success. Indeed, Pardo et al. [
19] reported cannibalism for this species in the wild, with 43% of exoskeleton crabs in the stomachs of early juveniles. In addition, Lovrich [
17] observed that newly molted juveniles of
L. santolla are eaten by adults. It has been observed in other juvenile crustaceans’ species that a higher risk of predation associated with complex habitats, creates a conspecific predation bottleneck [
69,
70]. Further evaluation of species’ habitats is needed to understand this particular characteristic, which may better describe the species’ population dynamics [
71].
In the present study, fragments of deteriorating algae had a relatively low volumetric contribution in the stomach content of
L. santolla and may represent a direct and/or indirect intake of other prey such as epibionts bryozoans, gastropods, etc. These results are different from those reported by Vinuesa et al. [
14], who observed a higher occurrence of red coralline algae on late juveniles of
L. santolla. Here, bivalves and crustaceans were of major importance in the stomach contents of late juveniles. This may suggest that the relative abundance of prey availability and their quality could account for omnivorous feeding at different trophic levels [
72,
73].
Additionally, we also recorded the presence of microplastics in the stomach content of
L. santolla, corresponding mainly to fibers between 0.05–5 mm in length. Fibers occur as both small strands, varying between blue and red, and in the form of a ball, ranging from transparent to black. Similar findings were reported by Andrade and Ovando [
74]. They point out that the probable origin of this waste and its transport to the sampling area is due to fishing activities, domestic use materials, and/or synthetic clothing. The possible routes of ingestion of the plastics can be by direct consumption or indirectly by trophic transfer from the lower trophic level in the food chain [
75]. Similar results of plastic ingestion have been found in other decapod crustaceans of commercial importance, such as Norway lobster
Nephrops norvegicus, where plastic fibers’ effects within the stomach contents caused a lower feeding rate and lower reserves [
76]. Recently, the presence of plastic fibers in the stomach contents of
Paralithodes camtschaticus has also been recorded, reaching 37.9% of 139 crabs analyzed [
26]. At the moment, the potential toxicity is unknown and the mechanical or other detrimental effects caused by the ingestion of plastics by
L. santolla or in the rest of the organisms that make up the food web in the Nassau Bay area.
For the first time, Cephalopoda were recorded in the stomach content of
L. santolla, since octopus beaks were found in it. In general, cephalopods are active predators of crustaceans, mollusks, polychaetes, fish, and other cephalopods and constitute an important carnivore in marine ecosystems [
77,
78]. In the Magellan sub-Antarctic region, the presence of dead octopuses on the seabed has been anecdotally documented by fishermen during diving activities (Eduardo Almonacid, 2019, personal communication.). Most probably,
L. santolla scavenges on octopus remnants, and such carrion prey availability can be beneficial to the
L. santolla.
When comparing the diet of
L. santolla to that of other decapod crustaceans from the northern hemisphere, similar findings are reported. For example, the opportunistic generalist Alaska red king crab
Paralithodes camtschaticus fed on 69 food items, with bivalves and polychaetes being the most consumed items for both late juveniles and adults, followed by echinoderms and crustaceans. The analysis also showed that all the specimens contained algae in their stomachs, but lower than other items [
26]. In addition, the omnivorous snow crab
Chionoecetes opilio’s mainly preys on polychaetes, mollusks, echinoderms, and teleost fishes, although the latter two in smaller quantities for both late juveniles and adults (30–130 mm CW) [
32].
4.2. Contribution of Kelp Carbon as the Major Source to Lithodes santolla
Our study significantly expands the knowledge on
L. santolla trophic ecology studies since the stable isotope analysis provided a novel insight into resource use within a population across sex and maturity stage groups, trophic niche, overlap, trophic position, and interactions with crab trap-associated fauna. There is very little isotopic information on this species [
19]. In general, the mean values of
δ13C and
δ15N of
L. santolla are similar to those found in the Patagonian fjord ecosystem for this species [
19,
79], and they are within the range found for other species of omnivore crabs from high latitude ecosystems [
26,
32].
The carbon signal of
L. santolla showed intermediate values in Nassau Bay, which reflected a nearshore ecosystem, where nearby kelp belts of
Macrocystis pyrifera provide a predictable source of nutrition. Conversely, the mean values of
δ13C found in the
L. santolla population presented a variation between –16.83‰ and –13.22‰, which corresponds to a food chain producer-based primary with a C
4 photosynthetic pathway (e.g., marine brown macroalgae [
80]). The Bayesian mixing models confirmed that
M. pyrifera was the most important basal carbon source for southern king crabs in Nassau Bay compared to the red algae
Porphyra columbina and the sediment, whose contribution seems to be little.
The incorporation of kelp carbon into
L. santolla body tissues may be from the direct and/or indirect consumption of
M. pyrifera. Consequently, the transfer of energy and nutrients is mediated by abundant primary consumers such as herbivores and grazers relying on feeding on kelp [
81,
82,
83]. Thus, the giant kelp
M. pyrifera would be an important carbon donor throughout the benthic food web [
84]. However, we cannot ignore that quantities of decomposed kelp material and macroalgal drift can be an important carbon source that enter the food chain through detrital pathways [
85,
86,
87]. To disentangle these mechanisms of energy pathways, further isotopic information is required from other allochthonous and autochthonous carbon food sources and from other organisms inhabiting and consuming
M. pyrifera in this high-latitude marine ecosystem. In addition, the spatio-temporal variability of basal sources should be considered, as their effects on the quantity/quality of available food for consumers are well known [
88,
89,
90,
91]. For instance, in sub-Antarctic marine systems, macroalgal carbon contributions sustain benthic food webs [
92,
93]. Large amounts of macroalgae-derived suspended particulate matter of kelp origin and resuspended kelp detritus represent significant nutrition sources for nearshore habitats [
93,
94]. The importance of kelp as an important trophic base for benthic food webs could also be due to the high polyunsaturated fatty-acid content [
95] and probably, being of nutritional importance for the growth and reproduction of invertebrates, as many algae had been described to serve as high-quality food for invertebrates in other systems [
96].
4.3. Intraspecific Niche Variation and Overlap of Lithodes santolla
The isotopic patterns observed show intraspecific trophic niche variation in the southern king crab. Niche size and region representations revealed a considerable dietary partitioning between sex and maturity, showing a greater tendency to forage different food items. It is perhaps forage intake that underpins differences in habitat use. The isotopic niches of individuals either overlapped or showed some levels of similar use and resource partitioning. The intraspecific niche variation observed in adult males/females indicated that their exploitation of trophic resources is more diversified than late juvenile males/females. These findings may be attributed to an ontogenetic shift linked to body size [
97,
98], age [
99], seasonal migration [
100], and sexual segregation [
101], among others. However, spatial separation by migratory patterns in the life cycle of
L. santolla [
44,
102,
103] reinforces the idea that the high mobility of adults from both sexes, when migrating from shallow to deep waters, means they have more access to habitat foraging resources than the juveniles do. Therefore, the niche differentiation found in the southern king crab population allows the species to partition resources since they likely share the same ecological niche, which facilitates the coexistence of similar species [
104]. The mechanism associated with this pattern in
L. santolla has yet to be explored.
The narrower niche found in late juveniles from both sexes could indicate limited access to forage by the availability of food in the habitat and therefore can aid resource partitioning and a more specialized diet [
105]. Consumer specialization shifts, for a particular resource or habitat use, are mostly related to ontogenetic changes in resource use in organisms [
105,
106]. For instance, evidence based on juvenile pods of
L. santolla around kelp beds of
Macrocystis pyrifera [
8] supports the idea of juvenile specialization on kelp-associated prey (e.g., bivalves). Moreover, kelp forests and beds of
M. pyrifera are common habitats, forming in shallow coastal ecosystems in the Magellan region [
63,
107], and smaller juveniles of
L. santolla seem to occupy them as microhabitats to minimize the risk of predation or cannibalism [
14].
Despite these findings, one also has to account for the influence when routing processes of isotopic data to estimate niche variation since outliers were included in our niche analysis to not underestimate the natural variability of organisms [
67] as we do not have isotopic prey availability data.
On the other hand, the significant enrichment in
δ15N found in adult males enables them to forage on food sources higher in
δ15N than females and late juveniles in various habitats, including kelp beds, along a spatial gradient and also on prey sources, thus increasing their trophic position. Besides, adult females showed more enrichment of
δ13C, suggesting they are using other food sources more related to coastal waters (e.g., littoral benthic environment). Larger niche sizes in adults may also indicate a shift from a foraging behavior to scavenging [
16], perhaps across a wide range of benthic habitats (e.g., nearshore and offshore). Given these characteristics in the complex feeding ecology of
L. santolla, it would be useful to understand how ontogenetic changes affect an organism’s resource use to optimize their energetic requirements and nutrition as they grow and when food resources are limited [
96].
The overlapping of Bayesian ellipses provides a measure of the diversity of individual patterns in resource use [
31] so that the results account for a broad use of trophic resources. There are similar resource requirements such as food and habitat, with varying degrees of overlap depending on the availability of the limited resources. It is possible that the overlapping of niches emphasizes the reported cannibalism for this species in their natural environment [
19]. Indeed, cannibalism has been described as a common phenomenon among crustaceans [
9,
108,
109].
4.4. Trophic Position of Lithodes santolla
According to our results,
L. santolla has an intermediate trophic position belonging to a secondary consumer (relative trophic level = ~3), and its range reflects an intraspecific variation in diet (i.e., trophic level does not relate to
δ13C), which supports an omnivore-based diet. Moreover, regression analyses indicated that as the
L. santolla increased its body size and body mass, the isotopic niche moved along both axes, with a significant shift from late juveniles to adults, towards more enriched
δ15N values reflected in a higher trophic position. Therefore, these results suggest that southern king crab tissues assimilate
13C- and
15N-enriched food sources as they grow and develop [
110,
111,
112]. Consequently, trophic positions increase with ontogeny. However, our data should be interpreted cautiously, as it does not fully reflect all
L. santolla life stages. Hence, an extended sampling that incorporates early juveniles should be carried out. Recently, a published paper by Pardo et al. [
20], focused on juveniles of
L. santolla, found an ontogenetic shift where the vagile phase occupied a higher trophic position than the cryptic phase. In general, ontogenetic niche shifts through life history are common in aquatic consumers [
97,
99,
113], particularly many crustaceans display ontogenetic dietary shifts related to body size increases [
114,
115,
116].
The estimated trophic position for the southern king crab in this study (3.3) is higher compared to that reported by Pardo et al. [
19] for other populations within the region, although the species showed a similar diet. These findings can be attributed to the basal carbon signature assimilated by
L. santolla and to the availability of high-quality food in the environment. Another comparison can be made with the red king crab
Paralithodes camtschaticus (3.1), a decapod from a different high latitude ecosystem [
26], wherein both species represent a trophic role of a secondary consumer, also called the first-order predator, in the trophic structure.
4.5. Community Niche Width
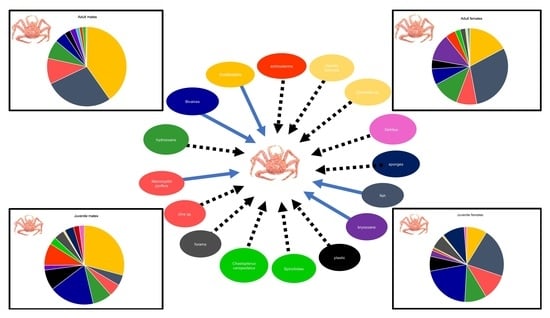
Our study represents the first attempt to estimate the trophic niche overlap of
L. santolla with associate fauna, as co-existing species, and their niche widths. Although there are many target fisheries in the area, the marine ecosystems and their trophic pathways’ functioning remains poorly understood. Stable isotope analyses indicated that
L. santolla overlap their isotopic niche, more or less, with some species of the associated fauna, such as false southern king crab, eelpout fish, and starfish, evidencing the sharing of food resources and habitat use. For example,
L. santolla may share resources and habitat with
P. granulosa. They present similar foraging behaviors and preys found in both species’ diets (e.g., algae, mollusks, crustaceans, and echinoderms) [
69]. The starfish, on the other hand, it may share gastropods and bivalves’ prey, but also microhabitat since it is actively feeding in kelp
M. pyrifera [
82]. For eelpout fish, there is no information about its diet and these results might suggest that this species is a potential predator of the southern king crab as seen by the niche overlap. Moreover, eelpout fish has a similar niche size with southern king crab and may suggest both species share food sources and habitats.
In contrast, there is no evidence in our study of niche overlap within the community between
L. santolla and the southern octopus and piquilhue snail. This is probably related to different habitats and preys, besides both species occupy a higher trophic position than
L. santolla. In addition, the narrow niche size found in piquilhue snail seems to be a more specialist diet. In fact, Bigatti et al. [
117] reported two dominant bivalve species in piquilhue snail stomach contents. Further research that includes more species interactions is necessary for improving the knowledge of the benthic food web.
The results of this study are relevant for ecosystem-based management in Nassau Bay. Therefore, it would be important to conduct long-term trophic studies to clarify whether the competing species are using the same habitats [
97] and what the overlap niche effects are of multiple target fisheries in the abundance of food source availability.