Pug-Headedness Anomaly in a Wild and Isolated Population of Native Mediterranean Trout Salmo trutta L., 1758 Complex (Osteichthyes: Salmonidae)
Abstract
:1. Introduction
2. Materials and Methods
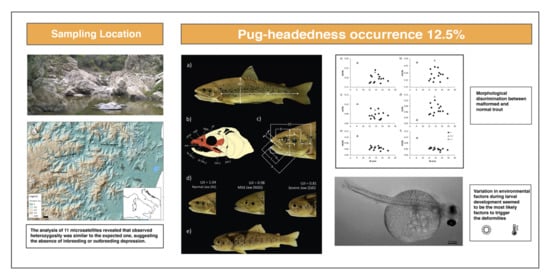
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Koumoundouros, G. Morpho-anatomical abnormalities in Mediterranean marine aquaculture. Recent Adv. Aquac. Res. 2010, 661, 125–148. [Google Scholar]
- Boglione, C.; Gavaia, P.; Koumoundouros, G.; Gisbert, E.; Moren, M.; Fontagné, S.; Witten, P.E. Skeletal anomalies in reared European fish larvae and juveniles. Part 1: Normal and anomalous skeletogenic processes. Rev. Aquac. 2013, 5, 99–120. [Google Scholar] [CrossRef] [Green Version]
- Boglione, C.; Gisbert, E.; Gavaia, P.; Witten, P.E.; Moren, M.; Fontagné, S.; Koumoundouros, G. Skeletal anomalies in reared European fish larvae and juveniles. Part 2: Main typologies, occurrences and causative factors. Rev. Aquac. 2013, 5, 121–167. [Google Scholar] [CrossRef] [Green Version]
- Boglione, C.; Costa, C.; Giganti, M.; Cecchetti, M.; Dato, P.; Di Scardi, M.; Cataudella, S. Biological monitoring of wild thicklip grey mullet (Chelon labrosus), golden grey mullet (Liza aurata), thinlip mullet (Liza ramada) and flathead mullet (Mugil cephalus) (Pisces: Mugilidae) from different Adriatic sites: Meristic counts and skeletal anoma. Ecol. Indic. 2006, 6, 712–732. [Google Scholar] [CrossRef] [Green Version]
- Jawad, L.A.; Ibrahim, M. Environmental oil pollution: A possible cause for the incidence of ankylosis, kyphosis, lordosis and scoliosis in five fish species collected from the vicinity of Jubail City, Saudi Arabia, Arabian Gulf. Int. J. Environ. Stud. 2018, 75, 425–442. [Google Scholar] [CrossRef]
- Gavaia, P.J.; Domingues, S.; Engrola, S.; Drake, P.; Sarasquete, C.; Dinis, M.T.; Cancela, M.L. Comparing skeletal development of wild and hatchery-reared Senegalese sole (Solea senegalensis, Kaup 1858): Evaluation in larval and postlarval stages. Aquac. Res. 2009, 40, 1585–1593. [Google Scholar] [CrossRef]
- Boglione, C.; Pulcini, D.; Scardi, M.; Palamara, E.; Russo, T.; Cataudella, S. Skeletal anomaly monitoring in rainbow trout (Oncorhynchus mykiss, Walbaum 1792) reared under different conditions. PLoS ONE 2014, 9, e96983. [Google Scholar] [CrossRef] [Green Version]
- Dawson, C. A bibliography of anomalies of fishes. Caribb. Res. Rep. 1964, 1, 308–399. [Google Scholar] [CrossRef]
- Shariff, M.; Zainuddin, A.T.; Abdullah, H. Pugheadedness in bighead carp, Aristichthys nobilis (Richardson). J. Fish Dis. 1986, 9, 457–460. [Google Scholar] [CrossRef]
- Pittman, K.; Bergh, O.I.; Skiftesvik, A.B.; Skjolddal, L.; Strand, H. Development of eggs and yolk sac larvae of halibut (Hippoglossus hippoglossus L.). J. Appl. Ichthyol. 1990, 6, 142–160. [Google Scholar] [CrossRef]
- Darias, M.J.; Mazurais, D.; Koumoundouros, G.; Le Gall, M.M.; Huelvan, C.; Desbruyeres, E.; Quazuguel, P.; Cahu, C.L.; Zambonino-Infante, J.L. Imbalanced dietary ascorbic acid alters molecular pathways involved in skeletogenesis of developing European sea bass (Dicentrarchus labrax). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 159, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.D.; Orth, D.J. First record of pughead deformity in blue catfish. Trans. Am. Fish. Soc. 2015, 144, 1111–1116. [Google Scholar] [CrossRef]
- Slooff, W. Skeletal anomalies in fish from. Aquat. Toxicol. 1982, 2, 157–173. [Google Scholar] [CrossRef]
- Eissa, A.E.; Moustafa, M.; El-Husseiny, I.N.; Saeid, S.; Saleh, O.; Borhan, T. Identification of some skeletal deformities in freshwater teleosts raised in Egyptian aquaculture. Chemosphere 2009, 77, 419–425. [Google Scholar] [CrossRef]
- Jawad, L.A.; Kousha, A.; Sambraus, F.; Fjelldal, P.G. On the record of pug-headedness in cultured Atlantic salmon, Salmo salar Linnaeus, 1758 (Salmoniformes, Salmonidae) from Norway. J. Appl. Ichthyol. 2014, 30, 537–539. [Google Scholar] [CrossRef]
- Jawad, L.; Thorsen, A.; Otterå, H.; Fjelldal, P. Pug-headedness in the farmed triploid Atlantic cod, Gadus morhua Linnaeus, 1758 (Actinopterygii: Gadiformes: Gadidae) in Norway. Acta Adriat. 2015, 56, 291–296. [Google Scholar]
- Catelani, P.A.; Bauer, A.B.; Di Dario, F.; Pelicice, F.M.; Petry, A.C. First record of pughead deformity in Cichla kelberi (Teleostei: Cichlidae), an invasive species in an estuarine system in south-eastern Brazil. J. Fish Biol. 2017, 90, 2496–2503. [Google Scholar] [CrossRef]
- Porta, M.J.; Snow, R.A. First record of pughead deformity in redear sunfish Lepomis microlophus (Günther, 1859). J. Appl. Ichthyol. 2019, 35, 775–778. [Google Scholar] [CrossRef]
- Bortone, S.A. Pugheadedness in the Pirate Perch, Aphredoderus sayanus (Pisces: Aphredoderidae), with Implications on Feeding. Chesap. Sci. 2006, 13, 231–232. [Google Scholar] [CrossRef]
- Bueno, L.S.; Koenig, C.C.; Hostim-Silva, M. First records of ‘pughead’ and ‘short-tail’ skeletal deformities in the Atlantic goliath grouper, Epinephelus itajara (Perciformes: Epinephelidae). Mar. Biodivers. Rec. 2015, 8, 10–13. [Google Scholar] [CrossRef]
- Grinstead, B.G. Effects of pigheadedness on growth and survival. Proc. Okla. Acad. Sci. 1971, 12, 8–12. [Google Scholar]
- Francini-Filho, R.B.; Amado-Filho, G.M. First record of pughead skeletal deformity in the queen angelfish Holacanthus ciliaris (St. Peter and St. Paul Archipelago, Mid Atlantic Ridge, Brazil). Coral Reefs 2013, 32, 211. [Google Scholar] [CrossRef] [Green Version]
- Franks, J.S. Food of Cobia, Rachycentron canadum, from the Northcentral Gulf of Mexico. Gulf Res. Rep. 1995, 9, 143–145. [Google Scholar]
- Jawad, L.; Hosie, A. On the record of pug-headedness in snapper, Pagrus auratus (Forster, 1801) (Perciformes, Sparidae) from New Zealand. Acta Adriat. 2007, 48, 205–210. [Google Scholar]
- Jawad, L.A.; Ibrahim, M. Head Deformity in Epinephelus diacanthus (Teleostei: Epinephelidae) and Oreochromis mossambicus (Teleostei: Cichlidae) Collected from Saudi Arabia and Oman, with a New Record of E. diacanthus to the Arabian Gulf Waters. Thalassas 2019, 35, 263–269. [Google Scholar] [CrossRef]
- Gudger, E.W. An adult pug-headed brown trout, Salmo fario: With notes on other pug-headed salmonids. Bull. Am. Mus. Nat. Hist. 1929, 58, 531–564. [Google Scholar]
- Splendiani, A.; Palmas, F.; Sabatini, A.; Barucchi, V.C. The name of the trout: Considerations on the taxonomic status of the Salmo trutta L., 1758 complex (Osteichthyes: Salmonidae) in Italy. Eur. Zool. J. 2019, 86, 432–442. [Google Scholar] [CrossRef] [Green Version]
- Rondinini, C.; Battistoni, A.; Peronace, V.; Teofili, C. Lista Rossa IUCN dei Vertebrati Italiani; IT Stamperia Romana: Rome, Italy, 2013; p. 54. Available online: http://www.iucn.it/liste-rosse-italiane.php (accessed on 1 September 2020).
- Sabatini, A.; Palmas, F.; Podda, C.; Frau, G.; Serra, M.; Musu, A.; Cani, M.V.; Righi, T.; Splendiani, A.; Caputo Barucchi, V. Carta Ittica Regionale. Parte 1. Tratti Montani; Con Appprofondimenti Sulla Distribuzione Della Trota Sarda Salmo Cettii, Rafinesque, 1810 Vol. 1. Tecnical Report; Regione Autonoma della Sardegna, Cagliari, Italy, Stamperia Regione Autonoma della Sardegna; 2018; p. 109. Available online: https://portalsardegnasira.it/-/carta-ittica-regionale-parte-i-tratti-momtani (accessed on 1 September 2020).
- Lockwood, R.N.; Schneider, J.C. Stream fish population esti- mates by mark- and-recapture and depletion methods. In Manual of Fisheries Survey Methods II: With Periodic Updates; Schneider, J.C., Ed.; Fisheries Special Report 25; Department for Natural Resources: Ann Arbor, MI, USA, 2000; pp. 1–14. [Google Scholar]
- Sušnik, S.; Snoj, A.; Dovč, P. Evolutionary distinctness of grayling (Thymallus thymallus) inhabiting the Adriatic river system, as based on mtDNA variation. Biol. J. Linn. Soc. 2001, 74, 375–385. [Google Scholar] [CrossRef] [Green Version]
- Bernatchez, L.; Danzmann, R.G. Congruence in Control-Region Sequence and Restriction-Site Variation in Mitochondrial DNA of Brook Charr (Salvelinus fontinalis Mitchill). Mol. Biol. Evol. 1993, 10, 1002–1014. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [Green Version]
- McMeel, O.M.; Hoey, E.M.; Ferguson, A. Partial nucleotide sequences, and routine typing by polymerase chain reaction-restriction fragment length polymorphism, of the brown trout (Salmo trutta) lactate dehydrogenase, LDH-C1*90 and *100 alleles. Mol. Ecol. 2001, 10, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Splendiani, A.; Giovannotti, M.; Righi, T.; Fioravanti, T.; Cerioni, P.N.; Lorenzoni, M.; Carosi, A.; La Porta, G.; Barucchi, V.C. Introgression despite protection: The case of native brown trout in Natura 2000 network in Italy. Conserv. Genet. 2019, 20, 343–356. [Google Scholar] [CrossRef]
- Splendiani, A.; Fioravanti, T.; Ruggeri, P.; Giovannotti, M.; Carosi, A.; Marconi, M.; Righi, T.; Nisi Cerioni, P.; Lorenzoni, M.; Caputo Barucchi, V. Life history and genetic characterisation of sea trout Salmo trutta in the Adriatic Sea. Freshw. Biol. 2020, 65, 460–473. [Google Scholar] [CrossRef]
- Excoffier, L.; Lisher, H.E.L. ARLEQUIN suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Res. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Goudet, J. FSTAT, a Program to Estimate and Test Gene Diversities and Fixation Indices, version 2.9. 3; Institute of Ecology: Lausanne, Switzerland, 2001. [Google Scholar]
- Jones, O.R.; Wang, J. COLONY: A program for parentage and sibship inference from multilocus genotype data. Mol. Ecol. Res. 2010, 10, 551–555. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar]
- Lijalad, M.; Powell, M.D. Effects of lower jaw deformity on swimming performance and recovery from exhaustive exercise in triploid and diploid Atlantic salmon Salmo salar L. Aquaculture 2009, 290, 145–154. [Google Scholar] [CrossRef]
- Delling, B.; Crivelli, A.J.; Rubin, J.F.; Berrebi, P. Morphological variation in hybrids between Salmo marmoratus, and alien Salmo species in the Volarja stream, Soca River basin, Slovenia. J. Fish. Biol. 2000, 57, 1199–1212. [Google Scholar]
- Rohlf, F.J. The tps series of software. Hystrix 2015, 26, 1–4. [Google Scholar]
- Bernatchez, L. The Evolutionary History of Brown Trout (Salmo Trutta L.) Inferred From Phylogeographic, Nested Clade, and Mismatch Analyses of Mitochondrial Dna Variation. Evolution 2001, 55, 351–379. [Google Scholar] [CrossRef]
- Berra, T.; Au, R.J. Incidence of Teratological Fishes from Cedar Fork Creek, Ohio. Ohio J. 1981, 81, 225–229. [Google Scholar]
- Abdel, I.; Abellan, E.; Lopez-Albors, O.; Valdés, P.; Nortes, M.J.; Garcia-Alcazar, A. Abnormalities in the juvenile stage of sea bass (Dicentrarchus labrax L.) reared at different temperatures: Types, prevalence and effect on growth. Aquac. Int. 2004, 12, 523–538. [Google Scholar] [CrossRef]
- Sabatini, A.; Cannas, R.; Follesa, M.C.; Palmas, F.; Manunza, A.; Matta, G.; Pendugiu, A.A.; Serra, P.; Cau, A. Genetic characterization and artificial reproduction attempt of endemic Sardinian trout Salmo trutta L., 1758 (Osteichthyes, Salmonidae): Experiences in captivity. Ital. J. Zool. 2011, 78, 20–26. [Google Scholar] [CrossRef]
- Sabatini, A.; Podda, C.; Frau, G.; Cani, M.V.; Musu, A.; Serra, M.; Palmas, F. Restoration of native Mediterranean brown trout Salmo cettii Rafinesque, 1810 (Actinopterygii: Salmonidae) populations using an electric barrier as a mitigation tool. Eur. Zool. J. 2018, 85, 138–150. [Google Scholar] [CrossRef] [Green Version]
- Zaccara, S.; Trasforini, S.; Antognazza, C.M.; Puzzi, C.; Britton, J.R.; Crosa, G. Morphological and genetic characterization of Sardinian trout Salmo cettii Rafinesque, 1810 and their conservation implications. Hydrobiologia 2015, 760, 205–223. [Google Scholar] [CrossRef]
- Berrebi, P.; Caputo Barucchi, V.; Splendiani, A.; Muracciole, S.; Sabatini, A.; Palmas, F.; Tougard, C.; Arculeo, M.; Marić, S. Brown trout (Salmo trutta L.) high genetic diversity around the Tyrrhenian Sea as revealed by nuclear and mitochondrial markers. Hydrobiologia 2018, 826, 209–231. [Google Scholar] [CrossRef]
- Delling, B.; Sabatini, A.; Muracciole, S.; Tougard, C.; Berrebi, P. Morphologic and genetic characterisation of Corsican and Sardinian trout with comments on Salmo taxonomy. Knowl. Manag. Aquat. Ecosyst. 2020, 421, 21. [Google Scholar] [CrossRef]
- Bruno, D.W.; Noguera, P.A.; Poppe, T.T. A Color Atlas Salmonid Diseases; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2013; p. 220. [Google Scholar]
- Damsgard, B.; Juell, J.; Braastad, B. Welfare in Farmed Fish; Report 5; Fiskeriforskning: Bergen, Norway, 2006. [Google Scholar]
- Villeneuve, L.; Gisbert, E.; Zambonino-Infante, J.L.; Quazuguel, P.; Cahu, C.L. Effect of nature of dietary lipids on European sea bass morphogenesis: Implication of retinoid receptors. Br. J. Nutr. 2005, 94, 877–884. [Google Scholar] [CrossRef]
- Peruzzi, S.; Puvanendran, V.; Riesen, G.; Seim, R.R.; Hagen, Ø.; Martínez-Llorens, S.; Falk-Petersen, I.B.; Fernandes, J.M.O.; Jobling, M. Growth and development of skeletal anomalies in diploid and triploid Atlantic salmon (Salmo salar) fed phosphorus-rich diets with fish meal and hydrolyzed fish protein. PLoS ONE 2018, 13, e0194340. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Harada, Y. Loss of genetic variation and effective population size of Kirikuchi charr: Implications for the management of small, isolated salmonid populations. Anim. Conserv. 2008, 11, 153–159. [Google Scholar] [CrossRef]
- Vincenzi, S.; Crivelli, A.J.; Jesenšek, D.; De Leo, G.A. The management of small, isolated salmonid populations: Do we have to fix it if it ain’t broken? Anim. Conserv. 2010, 13, 21–23. [Google Scholar] [CrossRef]
- Morita, K.; Yamamoto, S. Occurrence of a deformed white-spotted charr, Salvelinus leucomaenis (Pallas), population on the edge of its distribution. Fish. Manag. Ecol. 2000, 7, 551–553. [Google Scholar] [CrossRef]
- Sato, S. Occurrence of Deformed Fish and Their Fitness-related Traits in Kirikuchi Charr, Salvelinus ieucomaenis japonicus, the Southernmost Population of the Genus Salvelinus. Zool. Sci. 2006, 23, 593–599. [Google Scholar] [CrossRef] [PubMed]



| River | Sample Size | MtDNA | LDH-C1* | Microsatellite | FIS | ||||
|---|---|---|---|---|---|---|---|---|---|
| AD | ME | AT | *90 | *100 | HO (s.d.) | HE (s.d.) | |||
| Furittu | 16 | 1.00 | 1.00 | 0.50 (0.26) | 0.46 (0.14) | −0.089 | |||
| Neia | 22 | 0.45 | 0.32 | 0.23 | 0.77 | 0.23 | 0.80 (0.18) | 0.83 (0.20) | 0.051 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmas, F.; Righi, T.; Musu, A.; Frongia, C.; Podda, C.; Serra, M.; Splendiani, A.; Caputo Barucchi, V.; Sabatini, A. Pug-Headedness Anomaly in a Wild and Isolated Population of Native Mediterranean Trout Salmo trutta L., 1758 Complex (Osteichthyes: Salmonidae). Diversity 2020, 12, 353. https://doi.org/10.3390/d12090353
Palmas F, Righi T, Musu A, Frongia C, Podda C, Serra M, Splendiani A, Caputo Barucchi V, Sabatini A. Pug-Headedness Anomaly in a Wild and Isolated Population of Native Mediterranean Trout Salmo trutta L., 1758 Complex (Osteichthyes: Salmonidae). Diversity. 2020; 12(9):353. https://doi.org/10.3390/d12090353
Chicago/Turabian StylePalmas, Francesco, Tommaso Righi, Alessio Musu, Cheoma Frongia, Cinzia Podda, Melissa Serra, Andrea Splendiani, Vincenzo Caputo Barucchi, and Andrea Sabatini. 2020. "Pug-Headedness Anomaly in a Wild and Isolated Population of Native Mediterranean Trout Salmo trutta L., 1758 Complex (Osteichthyes: Salmonidae)" Diversity 12, no. 9: 353. https://doi.org/10.3390/d12090353