Endosymbiotic Green Algae in Paramecium bursaria: A New Isolation Method and a Simple Diagnostic PCR Approach for the Identification
Abstract
:1. Introduction
2. Material and Methods
2.1. Cultivation and Molecular Characterization of Paramecium bursaria
2.2. Isolation of the Green Algal Endosymbionts
2.3. Diagnostic PCR Amplification
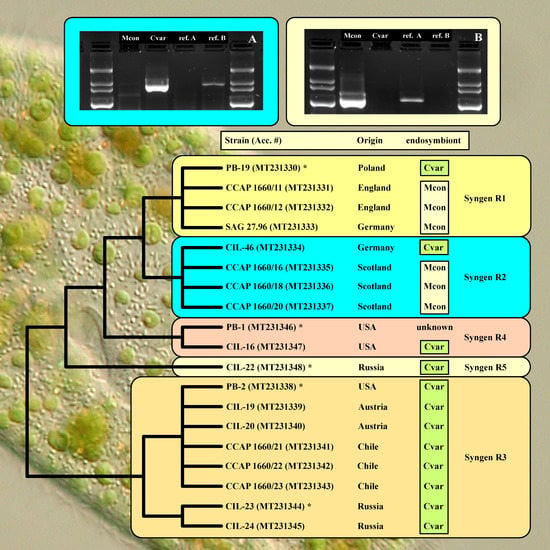
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pröschold, T.; Darienko, T.; Silva, P.C.; Reisser, W.; Krienitz, L. The systematics of “Zoochlorella” revisited employing an integrative approach. Environ. Microbiol. 2011, 13, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Muscatine, L.; Karakashian, S.J.; Karakashian, M.W. Soluble extracellular products of algae symbiotic with a ciliate, a sponge and a mutant Hydra. Comp. Biochem. Physiol. 1967, 20, 1–12. [Google Scholar] [CrossRef]
- Sommaruga, R.; Sonntag, B. Photobiological aspects of the mutualistic association between Paramecium bursaria and Chlorella. Microbiol. Monogr. 2009, 12, 111–130. [Google Scholar]
- Jeanniard, A.; Dunigan, D.D.; Gurnon, J.R.; Agarkova, I.V.; Kang, M.; Vitek, J.; Duncan, G.; McClung, O.W.; Larsen, M.; Claverie, J.-M.; et al. Towards defining the chloroviruses: A genomic journey through a genus of large DNA viruses. BMC Genom. 2013, 14, 158. [Google Scholar] [CrossRef] [Green Version]
- Foissner, W.; Berger, H.; Schaumburg, J. Identification and ecology of limnetic plankton ciliates. Bayer. Landesamt Wasserwirtsch. Munich Inf. 1999, 3/99, 1–793. [Google Scholar]
- Kreutz, M.; Foissner, W. The Sphagnum ponds of Simmelried in Germany: A biodiversity hot-spot for microscopic organisms. Protozool. Monogr. 2006, 3, 1–267. [Google Scholar]
- Lynn, D.H. The Ciliated Protozoa. Characterization, Classification, and Guide to the Literature; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2008. [Google Scholar]
- Hoshina, R.; Hayashi, S.; Imamura, N. Intraspecific genetic divergence of Paramecium bursaria and re-construction of the Paramecian phylogenetic tree. Acta Protozool. 2006, 45, 377–386. [Google Scholar]
- Greczek-Stachura, M.; Potekhin, A.; Przybos, E.; Rautian, M.; Skobio, I.; Tracz, S. Identification of Paramecium bursaria syngens through molecular markers—Comparative analysis of three loci in the nuclear and mitochondrial DNA. Protist 2012, 163, 671–685. [Google Scholar] [CrossRef]
- Fujishima, M. Endosymbionts in Paramecium. Microbiol. Monogr. 2009, 12, 1–252. [Google Scholar]
- Kreutz, M.; Stoeck, T.; Foissner, W. Morphological and molecular characterization of Paramecium (Viridoparamecium nov. subgen.) chlorelligerum Kahl 1935 (Ciliophora). J. Eukaryot. Microbiol. 2012, 59, 548–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, T. Simultaneous evaluation of life cycle dynamics between a host Paramecium and the endosymbionts of Paramecium bursaria using capillary flow cytometry. Sci. Rep. 2016, 6, 31638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pringsheim, E.G. Physiologische Untersuchungen an Paramecium bursaria: Ein Beitrag zur Symbioseforschung. Arch. Protistenkd. 1928, 64, 289–418. [Google Scholar]
- Loefer, J.B. Isolation and growth characteristics of the “Zoochlorella” of Paramecium bursaria. Am. Midl. Nat. 1936, 70, 184–188. [Google Scholar] [CrossRef]
- Gaponova, I.N.; Andronov, E.E.; Migunova, A.V.; Vorobyev, K.P.; Chizhevskaja, E.P.; Kvitko, K.V. Genomic dactyloscopy of Chlorella sp., symbionts of Paramecium bursaria. Protistology 2007, 4, 311–317. [Google Scholar]
- Hoshina, R.; Kamako, S.; Imamura, N. Phylogenetic position of endosymbiotic green algae in Paramecium bursaria Ehrenberg from Japan. Plant Biol. 2004, 6, 447–453. [Google Scholar] [CrossRef]
- Hoshina, R.; Kato, Y.; Kamako, S.; Imamura, N. Genetic evidence of “American” and “European” type symbiotic algae of Paramecium bursaria Ehrenberg. Plant Biol. 2005, 7, 526–532. [Google Scholar] [CrossRef]
- Hoshina, R.; Iwataki, M.; Imamura, N. Chlorella variabilis and Micractinium reisseri sp. nov. (Chlorellaceae, Trebouxiophyceae): Redescription of the endosymbiotic green algae of Paramecium bursaria (Peniculia, Oligohymenophorea) in the 120th year. Phycol. Res. 2010, 58, 188–201. [Google Scholar] [CrossRef]
- Hoshina, R.; Imamura, N. Multiple origins of the symbioses in Paramecium bursaria. Protist 2008, 159, 53–63. [Google Scholar] [CrossRef]
- Hoshina, R.; Imamura, N. Origins of algal symbionts of Paramecium bursaria. Microbiol. Monogr. 2009, 12, 1–29. [Google Scholar]
- Kang, M.; Dunigan, D.D.; Van Etten, J.L. Chlorovirus: A genus of Phycodnaviridae that infects certain Chlorella-like green algae. Mol. Plant Pathol. 2005, 6, 213–224. [Google Scholar] [CrossRef] [Green Version]
- Krienitz, L.; Bock, C. Present state of the systematics of planktonic coccoid green algae of inland waters. Hydrobiologia 2012, 698, 295–326. [Google Scholar] [CrossRef]
- Coleman, A.W. The significance of a coincidence between evolutionary landmarks found in mating affinity and a DNA sequence. Protist 2000, 151, 1–9. [Google Scholar] [CrossRef]
- Coleman, A.W. ITS2 is a double-edged tool for eukaryote evolutionary comparisons. Trends Genet. 2003, 19, 370–375. [Google Scholar] [CrossRef]
- Coleman, A.W. Pan-eukaryote ITS2 homologies revealed by RNA secondary structure. Nucleic Acid Res. 2007, 35, 3322–3329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bomford, R. The syngens of Paramecium bursaria: New mating types and intersyngenic mating reactions. J. Protozool. 1966, 13, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Schlösser, U.G. Additions to the culture collections of algae since 1994. Bot. Acta 1997, 110, 424–429. [Google Scholar] [CrossRef]
- Schlösser, U.G. SAG-Sammlung von Algenkulturen at the University of Göttingen. Bot. Acta 1994, 107, 424–429. [Google Scholar]
- Marin, B.; Palm, A.; Klingberg, M.; Melkonian, M. Phylogeny and taxonomic revision of plastid-containing euglenophytes based on SSU rDNA sequence comparisons and synapomorphic signatures in the SSU rRNA secondary structure. Protist 2003, 154, 99–145. [Google Scholar] [CrossRef]
- Swofford, D.L. PAUP* Phylogenetic Analysis Using Parsimony (*and other Methods), Version 4.0b10; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acid Res. 2003, 31, 3406–3615. [Google Scholar] [CrossRef]
- Byun, Y.; Han, K. PseudoViewer3: Generating planar drawings of large-scale RNA structures with pseudoknots. Bioinformatics 2009, 25, 1435–1437. [Google Scholar] [CrossRef]
- Shihira, I.; Krauss, R.W. Chlorella. Physiology and Taxonomy of Forty-One Isolates; University of Maryland: College Park, MD, USA, 1969. [Google Scholar]
- Kessler, E.; Huss, V.A.R. Biochemical taxonomy of symbiotic Chlorella strains from Paramecium and Acanthocystis. Bot. Acta 1990, 103, 140–142. [Google Scholar] [CrossRef]
- Siegel, R.W. Hereditary endosymbiosis in Paramecium bursaria. Exp. Cell Res. 1960, 19, 239–252. [Google Scholar] [CrossRef]
- Weis, D.S. Correlation of infectivity and Concanavalin A, agglutinability of algae exsymbiotic from Paramecium bursaria. J. Protozool. 1978, 25, 366–370. [Google Scholar] [CrossRef]
- Reisser, W. The endosymbiotic unit of Stentor polymorphus and Chlorella sp., morphological and physiological studies. Protoplasma 1981, 105, 273–284. [Google Scholar] [CrossRef]
- Nishihara, N.; Horiike, S.; Takahashi, T.; Kosaka, T.; Shigenaka, Y.; Hosoya, H. Cloning and characterization of endosymbiotic algae isolated from Paramecium bursaria. Protoplasma 1998, 203, 91–99. [Google Scholar] [CrossRef]
- Achilles-Day, U.E.M.; Day, J.G. Isolation of clonal cultures of endosymbiotic green algae from their ciliate hosts. J. Microbiol. Meth. 2013, 92, 355–357. [Google Scholar] [CrossRef]
- Jedlicki, A.; Fernández, G.; Astorga, M.; Oyarzún, P.; Toro, J.E.; Navarro, J.M.; Martinez, V. Molecular detection and species identification of Alexandrium (Dinophyceae) causing harmful algal blooms along the Chilean coastline. AoB Plants 2012, 2012, pls033. [Google Scholar] [CrossRef]
- Tanaka, M.; Murata-Hori, M.; Kadono, T.; Yamada, T.; Kawano, T.; Kosaka, T.; Hosoya, H. Complete elimination of endosymbiotic algae from Paramecium bursaria and its confirmation by diagnostic PCR. Acta Protozool. 2002, 41, 255–261. [Google Scholar]
- Summerer, M.; Sonntag, B.; Sommaruga, R. An experimental test of the symbiosis specificity between the ciliate Paramecium bursaria and strains of the unicellular green alga Chlorella. Environ. Microbiol. 2007, 9, 2117–2122. [Google Scholar] [CrossRef] [PubMed]
- Summerer, M.; Sonntag, B.; Sommaruga, R. Ciliate-symbiont specificity of freshwater endosymbiotic Chlorella (Trebouxiophyceae, Chlorophyta). J. Phycol. 2008, 44, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Blanc, G.; Duncan, G.; Agarkova, I.; Borodovsky, M.; Gurnon, J.; Kuo, A.; Lindquist, E.; Lucas, S.; Pangilinan, J.; Polle, J.; et al. The Chlorella variabilis NC64A genome reveals adaptation to photosymbiosis, coevolution with viruses, and cryptic sex. Plant Cell 2010, 22, 2943–2955. [Google Scholar] [CrossRef] [Green Version]
- Arriola, M.; Velmurugan, N.; Zhang, Y.; Plunkett, M.H.; Hondzo, H.; Barney, B.M. Genome sequences of Chlorella sorokiniana UTEX 1602 and Micractinium conductrix SAG 241.80: Implications to maltose excretion by a green alga. Plant J. 2018, 93, 566–586. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Guo, W.; Van Etten, J.L.; Mower, J.P. Multiple origins of endosymbionts in Chlorellaceae with no reductive effects on the plastid or mitochondrial genomes. Sci. Rep. 2017, 7, 10101. [Google Scholar] [CrossRef] [Green Version]






| Strain Number | Syngen | Origin | Accession Number | Endosymbiont |
|---|---|---|---|---|
| PB-19 | R1 | Poland: Biebrza National Park | MT231330 | Cvar |
| CCAP 1660/11 | R1 | England: Cambridge, Cavendish Pond | MT231331 | Mcon |
| CCAP 1660/12 | R1 | England: Cambridge, Cavendish Pond | MT231332 | Mcon |
| SAG 27.96 | R1 | Germany: Göttingen, pond in Old Botanical Garden | MT231333 | Mcon |
| CIL-46 | R2 | Germany: Seeburger See near Göttingen | MT231334 | Cvar |
| CCAP 1660/16 | R2 | Scotland: Loch Inverawe, Inverawe | MT231335 | Mcon |
| CCAP 1660/18 | R2 | Scotland: Loch Lily, Inverawe | MT231336 | Mcon |
| CCAP 1660/20 | R2 | Scotland: Loch Lily, Inverawe | MT231337 | Mcon |
| PB-2 | R3 | USA: Massachusetts, Boston | MT231338 | Cvar |
| CIL-19 | R3 | Austria: Piburger See | MT231339 | Cvar |
| CIL-20 | R3 | Austria: Wildbichl | MT231340 | Cvar |
| CCAP 1660/21 | R3 | Chile: Concepción, artificial pond at University campus | MT231341 | Cvar |
| CCAP 1660/22 | R3 | Chile: Concepción, artificial pond at University campus | MT231342 | Cvar |
| CCAP 1660/23 | R3 | Chile: Concepción, artificial pond at University campus | MT231343 | Cvar |
| CIL-23 | R3 | Russia: Khabarovsk region, Amur river | MT231344 | Cvar |
| CIL-24 | R3 | Russia: Primorie, Kiparisovo | MT231345 | Cvar |
| PB-1 | R4 | USA: Massachusetts, Boston | MT231346 | unknown |
| CIL-16 | R4 | USA: North Carolina, Burlington | MT231347 | Cvar |
| CIL-22 | R5 | Russia: Astrakhan Nature Reserve | MT231348 | Cvar |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spanner, C.; Darienko, T.; Biehler, T.; Sonntag, B.; Pröschold, T. Endosymbiotic Green Algae in Paramecium bursaria: A New Isolation Method and a Simple Diagnostic PCR Approach for the Identification. Diversity 2020, 12, 240. https://doi.org/10.3390/d12060240
Spanner C, Darienko T, Biehler T, Sonntag B, Pröschold T. Endosymbiotic Green Algae in Paramecium bursaria: A New Isolation Method and a Simple Diagnostic PCR Approach for the Identification. Diversity. 2020; 12(6):240. https://doi.org/10.3390/d12060240
Chicago/Turabian StyleSpanner, Christian, Tatyana Darienko, Tracy Biehler, Bettina Sonntag, and Thomas Pröschold. 2020. "Endosymbiotic Green Algae in Paramecium bursaria: A New Isolation Method and a Simple Diagnostic PCR Approach for the Identification" Diversity 12, no. 6: 240. https://doi.org/10.3390/d12060240