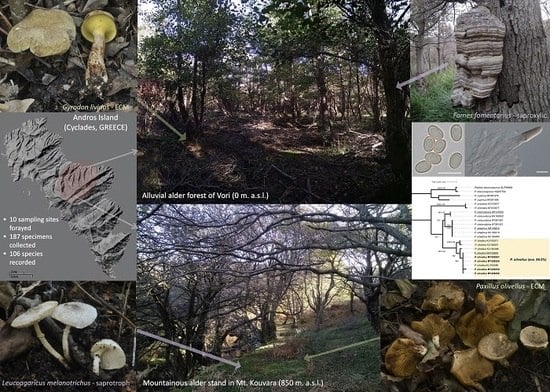
Basidiomycetes Associated with Alnus glutinosa Habitats in Andros Island (Cyclades, Greece)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling of Biological Material
2.2. Morpho-Anatomical Features in Basidiomes
2.3. DNA Extraction, Amplification and Sequencing
2.4. Phylogenetic Analysis of Sequence Data
3. Results and Discussion
3.1. The ECM Element
3.2. The Saproxylic Element
3.3. Litter and Other Terrestrial Decomposers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kajba, D.; Gračan, J. EuFORGEN Technical Guidelines for Genetic Conservation and Use for Black Alder (Alnus glutinosa); Bioversity International: Rome, Italy, 2003; pp. 1–6. [Google Scholar]
- Karl, T.R.; Trenberth, K.E. Modern Global Climate Change. Science 2003, 302, 1719–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEwan, N.R.; Wilkinson, T.; Girdwood, S.E.; Snelling, T.J.; Collins, T.; Dougal, K.; Jones, D.L.; Godbold, D.L. Evaluation of the microbiome of decaying alder nodules by next generation sequencing. Endocyt. Cell Res. 2017, 28, 14–19. [Google Scholar]
- Roy, M.; Pozzi, A.C.; Gareil, R.; Nagati, M.; Manzi, S.; Nouioui, I.; Sharikadze, N.; Jargeat, P.; Gryta, H.; Moreau, P.-A.; et al. Alder and the Golden Fleece: High diversity of Frankia and ectomycorrhizal fungi revealed from Alnus glutinosa subsp. barbata roots close to a Tertiary and glacial refugium. PeerJ 2017, 5, e3479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orfanoudakis, M.Z.; Hooker, J.E.; Wheeler-Jones, C.T. Early interactions between arbuscular mycorrhizal fungi and Frankia during colonisation and root nodulation of Alnus glutinosa. Symbiosis 2004, 36, 69–82. [Google Scholar]
- Põlme, S.; Öpik, M.; Moora, M.; Zobel, M.; Kohout, P.; Oja, J.; Kõljalg, U.; Tedersoo, L. Arbuscular mycorrhizal fungi associating with roots of Alnus and Rubus in Europe and the Middle East. Fungal Ecol. 2016, 24, 27–34. [Google Scholar] [CrossRef]
- Harley, J.L.; Smith, S.E. Mycorrhizal Symbiosis; Academic Press: London, UK, 1983; pp. 1–483. [Google Scholar]
- Pritsch, K.; Munch, J.C.; Buscot, F. Characterization and identification of black alder ectomycorrhizas by PCR/RFLP analyses of the rDNA internal described spacer (ITS). New Phytol. 1997, 137, 357–369. [Google Scholar] [CrossRef]
- Pritsch, K.; Munch, J.C.; Buscot, F. Morphological and anatomical characterisation of black alder Alnus glutinosa (L.) Gaertn. ectomycorrhizas. Mycorrhiza 1997, 7, 201–216. [Google Scholar] [CrossRef]
- Boyle, H. Aspekte der Macromycetenflora dreier Erlenbrücher Norddeutschlands und vergleichende PCR/RFLP- Analyse ausgewählter ectomycorrhizaler Mycobionten. EcoSys Suppl. 1996, 10, 1–106. [Google Scholar]
- Brunner, I.; Horak, E. Mycoecological analysis of Alnus associated macrofungi in the region of the Swiss National Park as recorded by J. Favre (1960). Mycol. Helv. 1990, 4, 111–139. [Google Scholar]
- Bujakiewicz, A.M. Macrofungi in the alder and alluvial forests in various parts of Europe and North America. Opera Bot. 1989, 100, 29–41. [Google Scholar]
- Kunttu, P.; Kotiranta, H.; Kulju, M.; Pasanen, H.; Kouki, J. Occurrence patterns, diversity and ecology of aphyllophoroid fungi on the black alder (Alnus glutinosa) in an archipelago in the Baltic Sea. Ann. Bot. Fenn. 2016, 53, 173–193. [Google Scholar] [CrossRef]
- Senn-Irlet, B.; Mürner, R.; Martini, E.; Küffer, N.; de Marchi, R.; Bieri, G. Saprobic fungi on wood and litter of Alnus alnobetula in the Swiss Alps. Mycotaxon 2012, 120, 506. [Google Scholar]
- Strid, Å. Wood-inhabiting fungi of alder forests in north-central Scandinavia 1. Aphylloporales (Basidiomycetes). Taxonomy, ecology and distribution. Wahlenbergia 1975, 1, 1–237. [Google Scholar]
- Griesser, B. Mykosoziologie der Grauerlen-und Sanddorn-Auen (Alnetum incanae, Hippophaëtum) am Hinterrhein (Domleschg, Graubünden, Schweiz). Ver. Geobot. Inst. ETH 1992, 109, 1–235. [Google Scholar]
- Molina, R. Ectomycorrhizal specificity in the genus Alnus. Can. J. Bot. 1981, 59, 325–334. [Google Scholar] [CrossRef]
- Tedersoo, L.; Suvi, T.; Jairus, T.; Ostonen, I.; Põlme, S. Revisiting ectomycorrhizal fungi of the genus Alnus: Differential host specificity, diversity and determinants of the fungal community. New Phytol. 2009, 182, 727–735. [Google Scholar] [CrossRef]
- Rochet, J.; Moreau, P.-A.; Manzi, S.; Gardes, M. Comparative phylogenies and host specialization in the alder ectomycorrhizal fungi Alnicola, Alpova and Lactarius (Basidiomycota) in Europe. BMC Evol. Biol. 2011, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Dimou, D.M.; Polemis, E.; Zervakis, G.I. Macromycetes associated with Alnus glutinosa in Greece. Phytopathol. Mediterr. 2006, 45, 78. [Google Scholar]
- Dimou, D.M.; Zervakis, G.I.; Polemis, E. Mycodiversity studies in selected ecosystems of Greece: IV. Macrofungi from Abies cephalonica forests and other intermixed tree species. (Oxya mountain, central Greece). Mycotaxon 2008, 104, 39–42. [Google Scholar]
- Polemis, E.; Dimou, D.M.; Tzanoudakis, D.; Zervakis, G.I. Diversity of Basidiomycota (subclass Agaricomycetidae) in the island of Andros (Cyclades, Greece). Nova Hedwig. 2012, 95, 25–58. [Google Scholar] [CrossRef]
- Polemis, E.; Dimou, D.; Zervakis, G.I. The family Hymenochaetaceae (Agaricomycetes, Basidiomycota) in the islands of the Aegean Archipelago (Greece). Plant Biosyst. 2013, 147, 306–314. [Google Scholar] [CrossRef]
- Polemis, E.; Roberts, P.; Dimou, D.M.; Zervakis, G.I. Heterobasidiomycetous fungi from Aegean Islands (Greece): New annotated records for a neglected group. Plant Biosyst. 2016, 150, 295–303. [Google Scholar] [CrossRef]
- Noordeloos, M.; Polemis, E. Studies in the genus Entoloma (Basidiomycetes, Agaricales) from the Kiklades (C. Aegean, Greece). Mycotaxon 2008, 105, 301–312. [Google Scholar]
- Eriksson, J.; Ryvarden, L. The Corticiaceae of North Europe, Vol. 4: Hyphodermella—Mycoacia; Fungiflora: Oslo, Norway, 1976; pp. 549–886. [Google Scholar]
- Bas, C.; Kuyper, T.W.; Noordeloos, M.E.; Vellinga, E.C. (Eds.) Flora Agaricina Neerlandica; A. A. Balkema: Rotterdam, The Netherlands, 1990; Volume 2, pp. 1–137. [Google Scholar]
- Bas, C.; Kuyper, T.W.; Noordeloos, M.E.; Vellinga, E.C. (Eds.) Flora Agaricina Neerlandica; A. A. Balkema: Rotterdam, The Netherlands, 1995; Volume 3, pp. 1–183. [Google Scholar]
- Bas, C.; Kuyper, T.W.; Noordeloos, M.E.; Vellinga, E.C. (Eds.) Flora Agaricina Neerlandica; A. A. Balkema: Rotterdam, The Netherlands, 1999; Volume 4, pp. 1–191. [Google Scholar]
- Noordeloos, M.E.; Kuyper, T.W.; Vellinga, E.C. (Eds.) Flora Agaricina Neerlandica; A. A. Balkema: Rotterdam, The Netherlands, 2001; Volume 5, pp. 1–170. [Google Scholar]
- Heilmann-Clausen, J.; Verbeken, A.; Vesterholt, J. The Genus Lactarius. Fungi of Northern Europe; Danish Mycological Society, Svampetryk: Copenhagen, Denmark, 1998; Volume 2, pp. 1–287. [Google Scholar]
- Bernicchia, A. Polyporaceae S.L.; Candusso: Alassio, Italy, 2005; pp. 1–808. [Google Scholar]
- Knudsen, H.; Vesterholt, J. (Eds.) Funga Nordica. Agaricoid, Boletoid and Cypheloid Genera; Nordsvamp: Copenhagen, Denmark, 2008; pp. 1–965. [Google Scholar]
- Antonín, V.; Noordeloos, M.E. A Monograph of Hemimycena, Delicatula, Fayodia, Gamundia, Myxomphalia, Resinomycena, Richenella and Pseudomphalina (Tribus Mycenae Sensu Singer, Mycena Excluded); IHW Verlag: Eching, Germany, 2004; pp. 1–279. [Google Scholar]
- Bernicchia, A.; Gorjón, S.P. Corticiaceae S.L.; Candusso: Alassio, Italy, 2010; pp. 1–1008. [Google Scholar]
- Aronsen, A.; Læssøe, T. The Genus Mycena. Fungi of Northern Europe; Svampetryk: Copenhagen, Denmark, 2016; Volume 5, pp. 1–373. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the RAxML Web servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.-A. A nomenclatural revision of the genus Alnicola (Cortinariaceae). Fungal Divers. 2005, 20, 121–155. [Google Scholar]
- Moreau, P.-A.; Peintner, U.; Gardes, M. Phylogeny of the ectomycorrhizal mushroom genus Alnicola (Basidiomycota, Cortinariaceae) based on rDNA sequences with special emphasis on host specificity and morphological characters. Mol. Phylogen. Evol. 2006, 38, 794–807. [Google Scholar] [CrossRef]
- De Haan, A.; Moreau, P.-A. Waarnemingen in het genus Alnicola (Zompzwam) in Vlaanderen (3). Steerbeckia 2012, 31, 3–15. [Google Scholar]
- Moser, M. Keys to Agarics and Boleti; R. Phillips: Tonbridge, UK, 1983; pp. 1–535. [Google Scholar]
- Horak, E. Röhrlinge und Blätterpilze in Europa—Unter der Mitarbeit von Anton Hausknecht (Bolbitiaceae) und P.A. Moreau (Alnicola); Elsevier Spektrum Akademischer: Heidelberg, Germany, 2005; pp. 1–557. [Google Scholar]
- Henrici, A. Keys to Naucoria in Britain. Field Mycol. 2009, 9, 55–62. [Google Scholar] [CrossRef]
- Jargeat, P.; Moreau, P.-A.; Gryta, H.; Chaumeton, J.P.; Gardes, M. Paxillus rubicundulus (Boletales, Paxillaceae) and two new alder-specific ectomycorrhizal species, Paxillus olivellus and Paxillus adelphus, from Europe and North Africa. Fungal Biol. 2016, 120, 711–728. [Google Scholar] [CrossRef]
- Wisitrassameewong, K.; Looney, B.P.; Le, H.T.; De Crop, E.; Das, K.; Van de Putte, K.; Eberhardt, U.; Jiayu, G.; Stubbe, D.; Hyde, K.D.; et al. Lactarius subgenus Russularia (Basidiomycota, Russulales): Novel Asian species, worldwide phylogeny and evolutionary relationships. Fungal Biol. 2016, 120, 1554–1581. [Google Scholar] [CrossRef] [PubMed]
- Galli, R. Le Russule. Atlante Pratico—Monografico per la Determinazione delle Russule; Dalla Natura: Milano, Italy, 2003; pp. 1–480. [Google Scholar]
- Floriani, M.; Partacini, G. Sull’identità di Russula puellaris var. leprosa. Boll. Gruppo Micol. G. Bres. 1998, 40, 213–218. [Google Scholar]
- Roy, M.; Rochet, J.; Manzi, S.; Jargeat, P.; Gryta, H.; Moreau, P.-A.; Gardes, M. What determines Alnus-associated ectomycorrhizal community diversity and specificity? A comparison of host and habitat effects at a regional scale. New Phytol. 2013, 198, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Breitenbach, J.; Kranzlin, F. Fungi of Switzerland; Verlag Mykologia: Lucerne, Switzerland, 2000; Volumes 1–5, pp. 1–342. [Google Scholar]
- Yurchenko, E.O. Natural substrata for corticioid fungi. Acta Mycol. 2006, 41, 113–124. [Google Scholar] [CrossRef]
- Ainsworth, M. Some British alder-associated wood- inhabiting fungi. Field Mycol. 2010, 11, 10–15. [Google Scholar] [CrossRef]
- Kotiranta, H.; Saarenoksa, R.; Kytövuori, I. Aphyllophoroid fungi of Finland. A check-list with ecology, distribution, and threat categories. Norrlinia 2009, 19, 1–223. [Google Scholar]
- Niemelä, T.; Kotiranta, H. Polypore survey of Finland 3. The genera Coltricia, Inonotopsis, Inonotus and Onnia. Karstenia 1983, 23, 15–25. [Google Scholar] [CrossRef]
- Küffer, N.; Senn-Irlet, B. Diversity and ecology of corticioid basidiomycetes in green alder stands in Switzerland. Nova Hedwig. 2000, 71, 131–143. [Google Scholar]
- Piętka, J.; Grzywacz, A. Grzyby wielkoowocnikowe stwierdzone na olszy czarnej Alnus glutinosa (L.) Gaertn. w drzewostanach olszowych wykazujących objawy zamierania. Sylwan 2018, 162, 22–31. [Google Scholar]
- Larsson, K.-H. Two new species in Hyphoderma. Nord. J. Bot. 1998, 18, 121–127. [Google Scholar] [CrossRef]
- Volobuev, S.; Okun, M.; Ordynets, A.; Spirin, V. The Phanerochaete sordida group (Polyporales, Basidiomycota) in temperate Eurasia, with a note on Phanerochaete pallida. Mycol. Prog. 2015, 14, 1–13. [Google Scholar] [CrossRef]
- Vellinga, E.C. Pluteaceae Kolt. & P. In Flora Agaricina Neerlandica; Bas, C., Noordeloos, M.E., Kuyper, T.W., Vellinga, E.C., Eds.; A. A. Balkema: Rotterdam, The Netherlands, 1990; Volume 2, pp. 31–64. [Google Scholar]
- Konstantinidis, G. 1000 Mushrooms of Western Makedonia; The Mushroom Friends Society of Western Makedonia: Kastoria, Greece, 2006; pp. 1–523. (In Greek) [Google Scholar]
- Ortega, A.; Esteve-Raventós, F. A new species of Gymnopilus (Cortinariaceae) from sandy soils in Pinus forests. Persoonia 2005, 18, 505–510. [Google Scholar]
- Guzman-Davalos, L.; Ortega, A.; Contu, M.; Vizzini, A.; Rodriguez, A.; Villalobos-Arambula, A.R.; Santerre, A. Gymnopilus maritimus (Basidiomycota, Agaricales), a new species from coastal psammophilous plant communities of northern Sardinia, Italy, and notes on G. arenophilus. Mycol. Prog. 2009, 8, 195–205. [Google Scholar] [CrossRef]
- Bas, C. Hydropus (Kühner) ex Singer. In Flora Agaricina Neerlandica; Bas, C., Kuyper, T.W., Noordeloos, M.E., Vellinga, E.C., Eds.; A. A. Balkema: Rotterdam, The Netherlands, 1999; Volume 4, pp. 166–172. [Google Scholar]
- Ortega, A.; Zea, M. Hydropus floccipes var. luteipes Ortega & Zea var. nov. en España meridional. Bol. Soc. Micol. Madr. 1991, 15, 189–191. [Google Scholar]
- Vellinga, E.C. Lepiota (Pers.: Fr.) S.F. Gray. In Flora Agaricina Neerlandica; Noordeloos, M.E., Kuyper, T.W., Vellinga, E.C., Eds.; A. A. Balkema: Rotterdam, The Netherlands, 2001; Volume 5, pp. 109–151. [Google Scholar]
- Gierczyk, B.; Kujawa, A.; Szczepkowski, A.; Chachuła, P. Rare species of Lepiota and related genera. Acta Mycol. 2011, 46, 137–178. [Google Scholar] [CrossRef]
- Noordeloos, M.E.; Bas, C. Flora Agaricina Neerlandica; Bas, C., Kuyper, T.W., Noordeloos, M.E., Vellinga, E.C., Eds.; A. A. Balkema: Rotterdam, The Netherlands, 1995; Volume 3, pp. 67–75. [Google Scholar]
- Deschuyteneer, D.; Melzer, A. Psathyrella hellebosensis, a new species from Belgium. Bull. AMFB 2017, 10, 3–10. [Google Scholar]
- Voto, P.; Dovana, F.; Garbelotto, M. A revision of the genus Psathyrella, with a focus on subsection Spadiceogriseae. FUSE 2019, 4, 97–170. [Google Scholar] [CrossRef] [PubMed]
- Noordeloos, M.E. Entoloma s.l. Supplemento. Fungi Europei Vol. 5a; Candusso: Alassio, Italy, 2004; pp. 1–760. [Google Scholar]
- Glassman, S.; Levine, C.; DiRocco, A.; Battles, J.J.; Bruns, T.D. Ectomycorrhizal fungal spore bank recovery after a severe forest fire: Some like it hot. ISME J. 2016, 10, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Zotti, M.; Persiani, A.M.; Ambrosio, E.; Vizzini, A.; Venturella, G.; Donnini, D.; Angelini, P.; Di Piazza, S.; Pavarino, M.; Lunghini, D.; et al. Macrofungi as ecosystem resources: Conservation versus exploitation. Plant Biosyst. 2013, 147, 219–225. [Google Scholar] [CrossRef] [Green Version]








| a/a | Species Name | Specimen Code/Collection Date | Locality | GenBank Accession No. |
|---|---|---|---|---|
| 1 | *Alnicola escharoides (Fr.) Romagn. | EP.17-A1344/11-Nov-2017 | Zenio | |
| EP.17-A1420/24-Nov-2017 | Vori | |||
| EP.18-A1548/22-Feb-2018 | Vori | MT458538 | ||
| EP.18-A1561/1-Nov-2018 | Zenio | MT458539 | ||
| EP.18-A1571/2-Nov-2018 | Vourkoti | MT458540 | ||
| EP.19-A1636/16 Nov 2019 | Katakalaioi | |||
| 2 | *Alnicola inculta (Peck) Singer | EP.17-A1346/11 Nov 2017 | Zenio | MT458541 |
| 3 | *Alnicola luteolofibrillosa Kühner | EP.17-A1430/24-Nov-2017 | Vori | MT458542 |
| 4 | *Alnicola subconspersa (Kühner ex P.D. Orton) Bon | EP.17-A1421/24-Nov-2017 | Vori | MT458543 |
| EP.19-A1637/16-Nov-2019 | Katakalaioi | MT458544 | ||
| 5 | Alnicola striatula (P.D. Orton) Romagn. | EP.04-A679/15-Nov-2004 | Evrousies | |
| EP.19-A1614/14-Nov-2019 | Evrousies | MT458545 | ||
| 6 | *Alnicola umbrina (R. Maire) Kühner | EP.04-A678/15-Nov-2004 | Evrousies | |
| EP.17-A1377/2-Nov-2017 | Lezina | MT458546 | ||
| EP.18-A1572/2-Nov-2018 | Vourkoti | MT458547 | ||
| EP.19-A1607/12 Nov 2019 | Zenio | MT458548 | ||
| EP.19-A1638/16-Nov-2019 | Katakalaioi | |||
| EP.19-A1646/17-Nov-2019 | Achlas riv. | |||
| EP.19-A1666/2-Dec-2019 | Remata | MT458549 | ||
| 7 | *Cortinarius americanus A.H. Sm. | EP.19-A1622/15-Nov-2019 | Vourkoti | |
| 8 | Gyrodon lividus (Bull.) Sacc. | EP.14-A1263/1-Nov-2014 | Vori | |
| EP.17-A1428/24-Nov-2017 | Vori | |||
| 9 | *Inocybe calospora Quél. | EP.18-A1570/2-Nov-2018 | Vourkoti | MT458550 |
| 10 | *Lactarius obscuratus (Lasch) Fr. | EP.17-A1347/11-Oct-2017 | Zenio | MT458551 |
| EP.17-A1566/1-Nov-2018 | Zenio | MT458552 | ||
| EP.17-A1576/2-Nov-2018 | Vourkoti | MT458553 | ||
| EP.19-A1645/17-Nov-2019 | Achlas riv. | MT458554 | ||
| EP.19-A1664/30-Nov-2019 | Remata | MT458555 | ||
| 11 | *Paxillus olivellus P.-A. Moreau, J.-P. Chaumeton, Gryta & Jarge | EP.95-A028/13-Nov-1995 | Achlas riv. | |
| EP.02-A353/22-Sep-2002 | Evrousies | |||
| EP.04-A670/23-Oct-2004 | Remata | |||
| EP.04-A673/24-Oct-2004 | Achlas riv. | |||
| EP.14-A1266/1-Nov-2014 | Vori | |||
| EP.17-A1348/11-Nov-2017 | Zenio | MT458556 | ||
| EP.17-A1396/23-Nov-2017 | Evrousies | MT458557 | ||
| EP.17-A1426/24-Nov-2017 | Vori | MT458558 | ||
| EP.18-A1552/22-Feb-2018 | Vori | MT458559 | ||
| EP.18-A1583/2-Nov-2018 | Vourkoti | |||
| EP.19-A1628/16-Nov-2019 | Katakalaioi | |||
| 12 | *Russula pumila Rouzeau & F. Massart | EP.18-A1575/2-Nov-2018 | Vourkoti | MT458560 |
| 13 | Tomentella stuposa (Link) Stalpers | EP.02-A327/29-Apr-2002 | Vori | MT458561 |
| 14 | Tomentella sublilacina (Ellis & Holw.) Wakef. | EP.02-A452/11-Oct-2002 | Achlas riv. | |
| EP.17-A1437/24-Nov-2017 | Vori | MT458562 |
| a/a | Species Name | Specimen Code/Collection Date | Locality | Substrate Type | GenBank Accession No. |
|---|---|---|---|---|---|
| 1 | Abortiporus biennis (Bull.) Singer | EP.18-A1582/02-Nov-2018 | Vourkoti | fallen trunk | |
| 2 | Agaricus moelleri Wasser | EP.19-A1613/14-Nov-2019 | Katakalaioi | leaf-litter | MT458502 |
| 3 | Amaropostia stiptica (Pers.) B.K. Cui, L.L. Shen & Y.C. Dai | EP.17-A1423/24-Nov-2017 | Vori | dead stump | MT458503 |
| 4 | Armillaria gallica Marxm. & Romagn. | EP.17-A1443/24-Nov-2017 | Vori | dead stump | |
| 5 | Armillaria mellea (Vahl) P. Kumm. | EP.95-A021/12-Nov-1995 | Remata | dead stump | |
| EP.18-A1584/02-Nov-2018 | Vourkoti | standing trunk | |||
| EP.19-A1651/29-Nov-2019 | Vori | trunk base | |||
| 6 | Auricularia auricula-judae (Bull.) Quél. | EP.18-A1539/22-Feb-2018 | Vori | standing trunk | |
| EP.19-A1672/02-Dec-2019 | Remata | standing trunk | |||
| 7 | Bjerkandera adusta (Willd.) P. Karst. | EP.19-A1670/02-Dec-2019 | Remata | fallen trunk | |
| 8 | Botryobasidium candicans J. Erikss. | EP.01-A275/26-Dec-2001 | Vori | fallen trunk | |
| EP.11-A1023/05-Jan-2011 | Vori | fallen trunk | |||
| EP.17-A1434/24-Nov-2017 | Vori | fallen trunk | |||
| 9 | Brevicellicium olivascens (Bres.) K.H. Larss. & Hjortstam | EP.17-A1352/11-Nov-2017 | Zenio | fallen trunk | MT458504 |
| 10 | Calocera cornea (Batsch) Fr. | EP.17-A1440/25-Nov-2017 | Vori | dead stump | |
| EP.19-A1616/14-Nov-2019 | Evrousies | dead stump | |||
| 11 | Ceriporia purpurea (Fr.) Donk | EP.17-A1350/11-Nov-2017 | Zenio | fallen trunk | MT458505 |
| EP.17-A1363/11-Nov-2017 | Zenio | fallen trunk | |||
| 12 | Chondrostereum purpureum (Pers.) Pouzar | EP.17-A1467/28-Nov-2017 | Achlas riv. | standing trunk | MT458506 |
| EP.19-A1678/02-Dec-2019 | Remata | standing trunk | |||
| 13 | Clavaria fragilis Holmsk. | EP.19-A1639/16-Nov-2019 | Katakalaioi | soil | |
| 14 | Clitocybe nebularis (Batsch) P. Kumm. | EP.18-A1580/02-Nov-2018 | Vourkoti | leaf litter | |
| 15 | Clitocybe phyllophila (Pers.) P. Kumm. | EP.18-A1579/02-Nov-2018 | Vourkoti | leaf litter | |
| 16 | Clitopilus hobsonii (Berk.) P.D.Orton | EP.02-A331/29-Apr-2002 | Vori | fallen trunk | |
| 17 | Coniophora puteana (Schumach.) P. Karst. | EP.11-A1017/5-Jan-2011 | Vori | fallen trunk | MT458507 |
| EP.17-A1411/24-Nov-2017 | Lefka | fallen trunk | MT458508 | ||
| EP.19-A1679/02-Dec-2019 | Remata | fallen trunk | MT458509 | ||
| 18 | Coprinellus disseminates (Pers.) J.E. Lange | EP.01-A260/26-Dec-2001 | Vori | rotten wood | |
| EP.19-A1654/29-Nov-2019 | Vori | around stump | |||
| 19 | Coprinellus radians (Fr.) Vilgalys, Hopple & Jacq. Johnson | EP.19-A1655/29-Nov-2019 | Vori | woody residues | MT458510 |
| EP.19-A1668/02-Dec-2019 | Remata | woody residues | |||
| 20 | *Coprinopsis melanthina (Fr.) Örstadius & E. Larss. | EP.17-A1412/24-Nov-2017 | Lefka | woody residues | MT458511 |
| EP.19-A1659/29-Nov-2019 | Vori | woody residues | |||
| 21 | Coriolopsis gallica (Fr.) Ryvarden | EP.11-A1016/05-Jan-2011 | Vori | standing trunk | |
| 22 | Crepidotus luteolus (Lambotte) Sacc. | EP.01-A268/26-Dec-2001 | Vori | woody residues | |
| 23 | Delicatula integrella (Pers.) Fayod | EP.19-A1634/16-Nov-2019 | Katakalaioi | trunk base | |
| EP.19-A1640/17-Nov-2019 | Achlas riv. | bark living tree | |||
| 24 | Entoloma alnicola Noordel. & Polemis | EP.02-A364/22-Sep-2002 | Evrousies | soil | |
| 25 | Entoloma incanum (Fr.) Hesler | EP.02-A362/22-Sep-2002 | Evrousies | soil | |
| EP.04-A674/24-Oct-2004 | Achlas riv. | soil | |||
| EP.19-A1633/16-Nov-2019 | Katakalaioi | soil | |||
| 26 | Entoloma juncinum (Kühner & Romagn.) Noordel. | EP.02-A362/22-Sep-2002 | Evrousies | soil | |
| 27 | Entoloma mougeotii (Fr.) Hesler | EP.02-A446/11-Oct-2002 | Achlas riv. | soil | |
| 28 | *Entoloma uranochroum Hauskn. & Noordel. | EP.19-A1615/14-Nov-2019 | Evrousies | soil/leaf-litter | |
| 29 | Exidiopsis galzinii (L.S. Olive) K. Wells | EP.02-A448/11-Oct-2002 | Achlas riv. | fallen trunk | |
| 30 | Fibroporia citrine (Bernicchia & Ryvarden) Bernicchia & Ryvarden | EP.17-A1356/11-Nov-2017 | Zenio | fallen trunk | |
| 31 | Fomes fomentarius (L.) Fr. | EP.20-A1681/05-Jan-2020 | Vori | standing trunk | |
| 32 | Fuscoporia torulosa (Pers.) T. Wagner | EP.04-A668/15-Oct-2004 | Evrousies | standing trunk | |
| EP.04-A672/24-Oct-2004 | Achlas riv. | standing trunk | |||
| EP.17-A1447/26-Nov-2017 | Achlas riv. | standing trunk | |||
| EP.19-A1677/02-Dec-2019 | Remata | standing trunk | |||
| 33 | Ganoderma adspersum (Schulzer) Donk | EP.14-A1264/01-Nov-2014 | Vori | standing trunk | |
| EP.17-A1422/24-Nov-2017 | Vori | standing trunk | |||
| 34 | Ganoderma resinaceum Boud. | EP.19-A1662/30-Nov-2019 | Remata | standing trunk | |
| 35 | *Gymnopilus arenophilus A. Ortega & Esteve-Rav. | EP.03-A659/04-Nov-2003 | Apoikia | rotten stump | |
| EP.17-A1408/24-Nov-2017 | Lefka | rotten stump | MT458512 | ||
| EP.19-A1674/02-Dec-2019 | Remata | rotten stump | MT458513 | ||
| 36 | Gymnopilus junonius (Fr.) P.D. Orton | EP.04-A667/15-Oct-2004 | Apoikia | standing trunk | |
| EP.04-A825/02-Dec-2004 | Apoikia | standing trunk | |||
| EP.17-A1385/14-Nov-2017 | Evrousies | standing trunk | |||
| EP.18-A1587/05-Nov-2018 | Vori | standing trunk | |||
| 37 | Gymnopus brassicolens (Romagn.) Antonín & Noordel. | EP.17-A1425/24-Nov-2017 | Vori | woody residues | |
| EP.19-A1673/02-Dec-2019 | Remata | woody residues | |||
| 38 | *Hydropus floccipes (Fr.) Singer | EP.19-A1658/29-Nov-2019 | Vori | standing trunk | |
| 39 | Hyphoderma medioburiense (Burt) Donk | EP.17-A1361/11-Nov-2017 | Zenio | fallen trunk | |
| EP.17-A1381/12-Nov-2017 | Katakalaioi | twigs | MT458514 | ||
| 40 | *Hyphoderma nemorale K.H. Larss. | EP.02-A326/29-Apr-2002 | Vori | fallen trunk | MT458515 |
| 41 | Hyphoderma setigerum (Fr.) Donk | EP.02-A332/29-Apr-2002 | Vori | fallen trunk | |
| EP.17-A1357/11-Nov-2017 | Zenio | fallen trunk | MT458516 | ||
| 42 | *Hyphodermella corrugate (Fr.) J. Erikss. & Ryvarden | EP.17-A1398/23-Nov-2017 | Evrousies | fallen branch | MT458517 |
| 43 | *Lepiota ochraceofulva P.D. Orton | EP.19-A1627/15-Nov-2019 | Vourkoti | leaf-litter | MT458518 |
| 44 | * Lepista ovispora (J.E. Lange) Gulden | EP.19-A1608/12-Nov-2019 | Zenio | leaf-litter | |
| 45 | Lepista nuda (Bull.) Cooke | EP.18-A1564/01-Nov-2018 | Zenio | leaf-litter | |
| 46 | Leucoagaricus melanotrichus (Malençon & Bertault) Trimbach | EP.19-A1604/12-Nov-2019 | Zenio | leaf-litter | |
| 47 | Leucopaxillus gentianeus (Quél.) Kotl. | EP.18-A1577/01-Nov-2018 | Zenio | leaf-litter | |
| 48 | Marasmius rotula (Scop.) Fr. | EP.02-A360/22-Sep-2002 | Evrousies | twigs | |
| 49 | Melanoleuca exscissa (Fr.) Singer | EP.17-A1415/24-Nov-2017 | Vori | soil | MT458519 |
| 50 | Merulius tremellosus Schrad. | EP.04-A680/15-Nov-2004 | Evrousies | fallen trunk | |
| EP.02-A323/29-Apr-2002 | Vori | fallen trunk | |||
| 51 | Mycena galericulata (Scop.) Gray | EP.17-A1349/11-Nov-2017 | Zenio | around trunk | |
| EP.19-A1625/15-Nov-2019 | Vourkoti | dead stump | MT458520 | ||
| EP.19-A1629/16-Nov-2019 | Katakalaioi | around trunk | MT458521 | ||
| EP.19-A1668/02-Dec-2019 | Remata | dead stump | |||
| 52 | Mycena haematopus (Pers.) P. Kumm. | EP.01-A262/26-Dec-2001 | Vori | rotten trunk | |
| 53 | Mycena pseudocorticola Kühner | EP.17-A1449/26-Nov-2017 | Achlas riv. | bark living tree | |
| EP.19-A1667/02-Dec-2019 | Remata | bark living tree | |||
| 54 | Mycena sanguinolenta (Alb. & Schwein.) P. Kumm | EP.19-A1642/17-Nov-2019 | Achlas riv. | twigs | |
| 55 | Mycetinis scorodonius (Fr.) A.W. Wilson & Desjardin | EP.19-A1644/17-Nov-2019 | Achlas riv. | twigs | |
| 56 | Mycoacia aurea (Fr.) J. Erikss. & Ryvarden | EP.02-A325/29-Apr-2002 | Vori | fallen trunk | |
| EP.11-A1026/05-Jan-2011 | Vori | fallen trunk | |||
| 57 | Mycoacia uda (Fr.) Donk | EP.17-A1351/11-Nov-2017 | Zenio | fallen trunk | |
| 58 | Mycoaciella bispora (Stalpers) Erikss. & Ryvarden | EP.01-A272/26-Dec-2001 | Vori | fallen trunk | |
| EP.02-A330/29-Apr-2002 | Vori | fallen trunk | |||
| 59 | Paralepista flaccida (Sowerby) Vizzini | EP.18-A1563/01-Nov-2018 | Zenio | leaf-litter | |
| EP.18-A1581/02-Nov-2018 | Vourkoti | leaf-litter | |||
| 60 | Parasola kuehneri (Uljé & Bas) Redhead, Vilgalys & Hopple | EP.18-A1546/22-Feb-2018 | Vori | soil | |
| 61 | Peniophora tamaricicola Boidin & Malenç. | EP.02-A329/29-Apr-2002 | Vori | fallen trunk | |
| 62 | Peniophorella praetermissa (P. Karst.) K.H. Larss. | EP.01-A276/26-Dec-2001 | Vori | fallen trunk | |
| EP.17-A1355/11-Nov-2017 | Zenio | fallen trunk | MT458522 | ||
| 63 | Perenniporia ochroleuca (Berk.) Ryvarden | EP.02-A461/11-Oct-2002 | Achlas riv. | dead wood | |
| 64 | *Phanerochaete livescens (P. Karst.) Volobuev & Spirin | EP.01-A273/26-Dec-2001 | Vori | fallen trunk | |
| EP.18-A1541/22-Feb-2018 | Vori | fallen trunk | MT458523 | ||
| 65 | Phellinus lundellii Niemelä | EP.17-A1342/13-Apr-2017 | Zenio | standing trunk | |
| 66 | Phlebia rufa (Pers.) M.P. Christ. | EP.17-A1354/11-Nov-2017 | Zenio | fallen trunk | MT458524 |
| EP.20-A1682/05-Jan-2020 | Vori | fallen trunk | MT458525 | ||
| 67 | Phlebiopsis ravenelii (Cooke) Hjortstam | EP.11-A1018/05-Jan-2011 | Vori | fallen trunk | |
| 68 | Phloeomana alba (Bres.) Redhead | EP.19-A1641/17-Nov-2019 | Achlas riv. | bark living tree | MT458526 |
| 69 | Phloeomana speirea (Fr.) Redhead | EP.17-A1378/12-Nov-2017 | Lezina | twigs | MT458527 |
| 70 | Physisporinus vitreus (Pers.) P. Karst. | EP.20-A1684/05-Jan-2020 | Vori | fallen trunk | |
| 71 | Pilatotrama ljubarskyi (Pilát) Zmitrovich | EP.18-A1551/22-Feb-2018 | Vori | fallen trunk | MT458528 |
| 72 | Pleurotus ostreatus (Jacq.) P. Kumm. | EP.01-A261/26-Dec-2001 | Vori | standing trunk | |
| EP.18-A1586/05-Nov-2018 | Vori | standing trunk | |||
| 73 | Pluteus cervinus (Schaeff.) P. Kumm. | EP.01-A321/29-Apr-2002 | Vori | dead wood | MT458529 |
| EP.19-A1623/15-Nov-2019 | Vourkoti | dead wood | MT458530 | ||
| EP.19-A1676/02-Dec-2019 | Remata | fallen trunk | |||
| 74 | Pluteus nanus (Pers.) P. Kumm. | EP.19-A1653/29-Nov-2019 | Vori | woody residues | |
| 75 | Pluteus salicinus (Pers.) P. Kumm. | EP.19-A1657/29-Nov-2019 | Vori | fallen branch | |
| 76 | *Pluteus podospileus Sacc. & Cub. | EP.19-A1643/17-Nov-2019 | Achlas riv. | fallen twigs | |
| 77 | Postia balsamea (Peck) Jülich | EP.19-A1663/30-Nov-2019 | Remata | fallen trunk | |
| 78 | Psathyrella candolleana (Fr.) Maire | EP.02-A336/04-Jun-2002 | Achlas riv. | woody residues | |
| EP.19-A1624/15-Nov-2019 | Vourkoti | woody residues | MT458531 | ||
| 79 | Psathyrella corrugis (Pers.) Konrad & Maubl. | EP.19-A1601/16-Oct-2019 | Vourkoti | soil/buried wood | MT458532 |
| 80 | *Psathyrella hellebosensis Deschuyteneer & A. Melzer | EP.17-A1409/24-Nov-2017 | Lefka | woody residues | MT458533 |
| 81 | Psathyrella microrhiza (Lasch) Konrad & Maubl. | EP.19-A1603/12-Nov-2019 | Vourkoti | woody residues | MT458534 |
| 82 | Psathyrella prona (Fr.) Gill. | EP.02-A363/22-Sep-2002 | Evrousies | soil | |
| 83 | Psathyrella vinosofulva P.D. Orton | EP.17-A1431/24-Nov-2017 | Vori | soil | MT458535 |
| 84 | Radulomyces confluens (Fr.) M.P. Christ. | EP.11-A1022/05-Jan-2011 | Vori | fallen trunk | |
| 85 | Steccherinum ochraceum (Pers. ex J.F. Gmel.) Gray | EP.18-A1540/22-Feb-2018 | Vori | fallen trunk | |
| 86 | Stereum hirsutum (Willd.) Pers. | EP.17-A1397/23-Nov-2017 | Evrousies | trunk/branch | |
| EP.17-A1463/28-Nov-2017 | Achlas riv. | trunk/branch | |||
| EP.18-A1544/22-Feb-2018 | Vori | trunk | |||
| EP.20-A1691/25-Jan-2020 | Lefka | branch | |||
| 87 | Trechispora nivea (Pers.) K.H. Larss. | EP.02-A328/29-Apr-2002 | Vori | fallen trunk | |
| EP.20-A1683/05-Jan-2020 | Vori | fallen trunk | MT458536 | ||
| 88 | Trametes versicolor (L.) Lloyd | EP.11-A1019/05-Jan-2011 | Vori | fallen trunk | |
| EP.18-A1542/22-Feb-2018 | Vori | standing trunk | |||
| 89 | Tubaria furfuracea (Pers.) Gillet | EP.19-A1652/29-Nov-2019 | Vori | woody residues | |
| 90 | Tulostoma fimbriatum Fr. | EP.17-A1431/24-Nov-2017 | Vori | soil | |
| 91 | Vitreoporus dichrous (Fr.) Zmitr. | EP.02-A445/11-Oct-2002 | Achlas riv. | fallen trunk | |
| EP.17-A1439/25-Nov-2017 | Vori | fallen branch | |||
| EP.18-A1538/22-Feb-2017 | Vori | fallen branch | |||
| 92 | Xylodon raduloides Riebesehl & Langer | EP.11-A1025/05-Jan-2011 | Vori | fallen trunk | |
| EP.18-A1543/22-Feb-2018 | Vori | fallen trunk | MT458538 | ||
| EP.19-A1671/02-Dec-2019 | Remata | fallen trunk |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polemis, E.; Fryssouli, V.; Daskalopoulos, V.; Zervakis, G.I. Basidiomycetes Associated with Alnus glutinosa Habitats in Andros Island (Cyclades, Greece). Diversity 2020, 12, 232. https://doi.org/10.3390/d12060232
Polemis E, Fryssouli V, Daskalopoulos V, Zervakis GI. Basidiomycetes Associated with Alnus glutinosa Habitats in Andros Island (Cyclades, Greece). Diversity. 2020; 12(6):232. https://doi.org/10.3390/d12060232
Chicago/Turabian StylePolemis, Elias, Vassiliki Fryssouli, Vassileios Daskalopoulos, and Georgios I. Zervakis. 2020. "Basidiomycetes Associated with Alnus glutinosa Habitats in Andros Island (Cyclades, Greece)" Diversity 12, no. 6: 232. https://doi.org/10.3390/d12060232