A New Genus of Terrestrial-Breeding Frogs (Holoadeninae, Strabomantidae, Terrarana) from Southern Peru
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Work
2.2. Molecular Phylogenetic Analyses
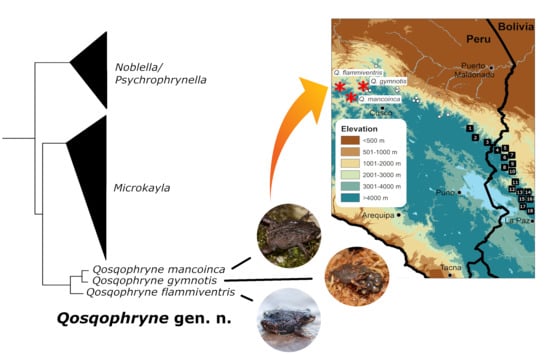
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Specimens Examined
References
- Hedges, S.B.; Duellman, W.E.; Heinicke, M.P. New World direct-developing frogs (Anura: Terrarana): Molecular phylogeny, classification, biogeography, and conservation. Zootaxa 2008, 1737, 1–182. [Google Scholar] [CrossRef] [Green Version]
- De La Riva, I.; Chaparro, J.C.; Castroviejo-Fisher, S.; Padial, J.M. Underestimated anuran radiations in the high Andes: Five new species and a new genus of Holoadeninae, and their phylogenetic relationships (Anura: Craugastoridae). Zool. J. Linn. Soc. 2018, 182, 129–172. [Google Scholar] [CrossRef]
- von May, R.; Lehr, E.; Rabosky, D.L. Evolutionary radiation of earless frogs in the Andes: Molecular phylogenetics and habitat shifts in high-elevation terrestrial breeding frogs. PeerJ 2018, 6, e4313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De La Riva, I. Unexpected Beta-Diversity Radiations in Highland Clades of Andean Terraranae. In Neotropical Diversification: Patterns and Processes; Rull, V., Carnaval, A., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Duellman, W.E.; Lehr, E. Terrestrial-Breeding Frogs (Strabomantidae) in Peru; Natur und Tier Verlag: Münster, Germany, 2009; p. 382. [Google Scholar]
- Heinicke, M.P.; Duellman, W.E.; Trueb, L.; Means, D.B.; MacCulloch, R.D.; Hedges, S.B. A new frog family (Anura: Terrarana) from South America and an expanded direct-developing clade revealed by molecular phylogeny. Zootaxa 2009, 2211, 1–35. [Google Scholar] [CrossRef]
- Heinicke, M.P.; Lemmon, A.R.; Lemmon, E.M.; McGrath, K.; Hedges, S.B. Phylogenomic support for evolutionary relationships of New World direct-developing frogs (Anura: Terraranae). Mol. Phylogenet. Evol. 2017. [Google Scholar] [CrossRef]
- Frost, D.R. Amphibian Species of the World: An Online Reference. Version 6.0. Available online: http://research.amnh.org/herpetology/amphibia/index.html (accessed on 23 October 2019).
- Heinicke, M.P.; Duellman, W.E.; Hedges, S.B. Major Caribbean and Central American frog faunas originated by ancient oceanic dispersal. Proc. Natl. Acad. Sci. USA 2007, 104, 10092–10097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehr, E.; von May, R. A new species of terrestrial-breeding frog (Amphibia, Craugastoridae, Pristimantis) from high elevations of the Pui Pui Protected Forest in central Peru. ZooKeys 2017, 660, 17–42. [Google Scholar] [CrossRef] [Green Version]
- Condori Ccarhuarupay, F.P. Filogenia Morfológica del Género Bryophryne Hedges, 2008 (Anura: Craugastoridae); Universidad Nacional San Antonio Abad del Cusco: Cusco, Peru, 2018. [Google Scholar]
- Chaparro, J.C.; Padial, J.M.; Gutierrez, R.C.; De la Riva, I. A new species of Andean frog of the genus Bryophryne from southern Peru (Anura: Craugastoridae) and its phylogenetic position, with notes on the diversity of the genus. Zootaxa 2015, 3994, 94–108. [Google Scholar] [CrossRef] [Green Version]
- Lehr, E.; Catenazzi, A. A new species of Bryophryne (Anura: Strabomantidae) from southern Peru. Zootaxa 2008, 1784, 1–10. [Google Scholar] [CrossRef]
- Lehr, E.; Catenazzi, A. Three new species of Bryophryne (Anura: Strabomantidae) from the Region of Cusco, Peru. S. Am. J. Herpetol. 2009, 4, 125–138. [Google Scholar] [CrossRef]
- Lehr, E.; Catenazzi, A. Two new species of Bryophryne (Anura: Strabomantidae) from high elevations in southern Peru (Region of Cusco). Herpetologica 2010, 66, 308–319. [Google Scholar] [CrossRef]
- Catenazzi, A.; Ttito, A.; Diaz, M.I.; Shepack, A. Bryophryne phuyuhampatu sp n. a new species of Cusco Andes frog from the cloud forest of the eastern slopes of the Peruvian Andes (Amphibia, Anura, Craugastoridae). Zookeys 2017, 685, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Mamani, L.; Catenazzi, A.; Ttito, A.; Mallqui, S.; Chaparro, J.C. A new species of Bryophryne (Anura: Strabomantidae) from the Cordillera de Vilcabamba, southeastern Peruvian Andes. Phyllomedusa 2017, 16, 129–141. [Google Scholar] [CrossRef] [Green Version]
- Chaparro, J.C.; De la Riva, I.; Padial, J.M.; Ochoa, J.A.; Lehr, E. A new species of Phrynopus from Departamento Cusco, southern Peru (Anura: Brachycephalidae). Zootaxa 2007, 1618, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Catenazzi, A.; Ttito, A. A new species of Psychrophrynella (Amphibia, Anura, Craugastoridae) from the humid montane forests of Cusco, eastern slopes of the Peruvian Andes. PeerJ 2016, 4, e1807. [Google Scholar] [CrossRef] [Green Version]
- Catenazzi, A.; Ttito, A. Psychrophrynella glauca sp. n. a new species of terrestrial-breeding frogs (Amphibia, Anura, Strabomantidae) from the montane forests of the Amazonian Andes of Puno, Peru. PeerJ 2018, 6, e444. [Google Scholar] [CrossRef] [Green Version]
- Catenazzi, A.; Uscapi, V.; von May, R. A new species of Noblella from the humid montane forests of Cusco, Peru. Zookeys 2015, 516, 71–84. [Google Scholar] [CrossRef]
- von May, R.; Catenazzi, A.; Corl, A.; Santa-Cruz, R.; Carnaval, A.C.; Moritz, C. Divergence of thermal physiological traits in terrestrial breeding frogs along a tropical elevational gradient. Ecol. Evol. 2017, 7, 3257–3267. [Google Scholar] [CrossRef]
- Canedo, C.; Haddad, C.F. Phylogenetic relationships within anuran clade Terrarana, with emphasis on the placement of Brazilian Atlantic rainforest frogs genus Ischnocnema (Anura: Brachycephalidae). Mol. Phylogenet. Evol. 2012, 65, 610–620. [Google Scholar] [CrossRef]
- Padial, J.M.; Grant, T.; Frost, D.R. Molecular systematics of terraranas (Anura: Brachycephaloidea) with an assessment of the effects of alignment and optimality criteria. Zootaxa 2014, 3825, 1–132. [Google Scholar] [CrossRef] [Green Version]
- Motta, A.P.; Chaparro, J.C.; Pombal, J.P.; Guayasamin, J.M.; De la Riva, I.; Padial, J.M. Molecular phylogenetics and taxonomy of the Andean genus Lynchius Hedges, Duellman, and Heinicke 2008 (Anura: Craugastoridae). Herpetol. Monogr. 2016, 30, 119–142. [Google Scholar] [CrossRef] [Green Version]
- Padial, J.M.; Chaparro, J.C.; Castroviejo-Fisher, S.; Guayasamin, J.M.; Lehr, E.; Delgado, A.J.; Vaira, M.; Teixeira, M., Jr.; Aguay, R.; de la Riva, I. A revision of species diversity in the Neotropical genus Oreobates (Anura: Strabomantidae), with the description of three new species from the Amazonian slopes of the Andes. Am. Mus. Novit. 2012, 3752, 1–55. [Google Scholar] [CrossRef] [Green Version]
- Santa-Cruz, R.; von May, R.; Catenazzi, A.; Whitcher, C.; Tejeda, E.L.; Rabosky, D.L. A new species of terrestrial-breeding frog (Amphibia, Strabomantidae, Noblella) from the upper Madre de Dios watershed, Amazonian Andes and lowlands of southern Peru. Diversity (Basel) 2019, 11, 145. [Google Scholar] [CrossRef] [Green Version]
- Lehr, E.; Fritzsch, G.; Müller, A. Analysis of Andes frogs (Phrynopus, Leptodactylidae, Anura) phylogeny based on 12S and 16S mitochondrial rDNA sequences. Zool. Scr. 2005, 34, 593–603. [Google Scholar] [CrossRef]
- Padial, J.M.; Chaparro, J.C.; De La Riva, I. Systematics of Oreobates and the Eleutherodactylus discoidalis species group (Amphibia, Anura), based on two mitochondrial DNA genes and external morphology. Zool. J. Linn. Soc. 2008, 152, 737–773. [Google Scholar] [CrossRef]
- Shepack, A.; von May, R.; Ttito, A.; Catenazzi, A. A new species of Pristimantis (Amphibia, Anura, Craugastoridae) from the foothills of the Andes in Manu National Park, southeastern Peru. Zookeys 2016, 574, 143–164. [Google Scholar]
- von May, R.; Rabosky, D.L.; Lehr, E. Earless frogs in the Andes: Extraordinary ecological divergence and morphological diversity. Nat. Hist. 2018, 126, 12–15. [Google Scholar]
- Palumbi, S.R.; Martin, A.; Romano, S.; McMillan, W.O.; Stice, L.; Grabawski, G. The Simple Fool’s Guide to PCR (Version 2.0); Privately published; Palumbi, S., Ed.; University of Hawaii: Honolulu, HI, USA, 1991. [Google Scholar]
- Meyer, C.P. Molecular systematics of cowries (Gastropoda: Cypraeidae) and diversification patterns in the tropics. Biol. J. Linn. Soc. 2003, 79, 401–459. [Google Scholar] [CrossRef] [Green Version]
- Bossuyt, F.; Milinkovitch, M.C. Convergent adaptive radiations in Madagascan and Asian ranid frogs reveal covariation between larval and adult traits. Proc. Natl. Acad. Sci. USA 2000, 97, 6585–6590. [Google Scholar] [CrossRef] [Green Version]
- Lanfear, R.; Calcott, B.; Ho, S.; Guindon, S. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef] [Green Version]
- Ronquist, F.; Huelsenbeck, J. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambaut, A.; Drummond, A. Tracer. Version 1.5. 2007. Available online: http://tree.bio.ed.ac.uk/software/tracer (accessed on 30 October 2019).
- Rambaut, A. FigTree. Version 1.4.2. 2009. Available online: http://tree.bio.ed.ac.uk/software/figtree (accessed on 30 October 2019).
- Mamani, L.; Diaz, M.I.; Ttito, J.W.; Condori, F.P.; Ttito, A. Parental care and altitudinal range extension of the endemic frog Bryophryne gymnotis (Anura: Craugastoridae) in the Andes of southeastern Peru. Phyllomedusa 2017, 16, 109–112. [Google Scholar] [CrossRef] [Green Version]
- Catenazzi, A.; Ttito, A. Noblella thiuni sp. n. a new (singleton) species of minute terrestrial-breeding frog (Amphibia, Anura, Strabomantidae) from the montane forest of the Amazonian Andes of Puno, Peru. PeerJ 2019, 7, e6780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes-Puig, J.P.; Reyes-Puig, C.; Ron, S.; Ortega, J.A.; Guayasamin, J.M.; Goodrum, M.; Recalde, F.; Vieira, J.J.; Koch, C.; Yanez-Munoz, M.H. A new species of terrestrial frog of the genus Noblella Barbour, 1930 (Amphibia: Strabomantidae) from the Llanganates-Sangay Ecological Corridor, Tungurahua, Ecuador. PeerJ 2019, 7, e7405. [Google Scholar] [CrossRef] [Green Version]
- De la Riva, I.; Chaparro, J.C.; Padial, J.M. The taxonomic status of Phyllonastes Heyer and Phrynopus peruvianus (Noble) (Lissamphibia, Anura): Resurrection of Noblella Barbour. Zootaxa 2008, 1685, 67–68. [Google Scholar] [CrossRef] [Green Version]
- Catenazzi, A.; von May, R. Conservation status of amphibians in Peru. Herpetol. Monogr. 2014, 28, 1–23. [Google Scholar] [CrossRef]
- De La Riva, I.; Reichle, S. Diversity and conservation of the amphibians of Bolivia. Herpetol. Monogr. 2014, 28, 46–65. [Google Scholar] [CrossRef] [Green Version]
- Catenazzi, A.; Lehr, E.; Rodriguez, L.O.; Vredenburg, V.T. Batrachochytrium dendrobatidis and the collapse of anuran species richness and abundance in the upper Manu National Park, southeastern Peru. Conserv. Biol. 2011, 25, 382–391. [Google Scholar] [CrossRef]
- Catenazzi, A.; Lehr, E.; Vredenburg, V.T. Thermal physiology, disease and amphibian declines in the eastern slopes of the Andes. Conserv. Biol. 2014, 28, 509–517. [Google Scholar] [CrossRef]
- Catenazzi, A.; Finkle, J.; Foreyt, E.; Wyman, L.; Swei, A.; Vredenburg, V.T. Epizootic to enzootic transition of a fungal disease in tropical Andean frogs: Are surviving species still susceptible? PLoS ONE 2017, 12, e0186478. [Google Scholar] [CrossRef]
- ICZN. International Code of Zoological Nomenclature, 4th ed.; International Trust for Zoological Nomenclature: London, UK, 1999. [Google Scholar]
- De la Riva, I. Bolivian frogs of the genus Phrynopus, with the description of twelve new species (Anura: Brachycephalidae). Herpetol. Monogr. 2007, 21, 241–277. [Google Scholar] [CrossRef]
- Willaert, B.; Reichle, S.; Stegen, G.; Martel, A.; Barrón, S.; Sánchez de Lozada, N.; Greenhawk, N.; Agostini, G.; Muñoz, A. Distribution, ecology, and conservation of the critically endangered frog Psychrophrynella illimani (Anura: Craugastoridae) with description of its call. Salamandra 2016, 52, 317–327. [Google Scholar]
- De la Riva, I.; Burrowes, P.A. A new species of Psychrophrynella (Anura: Craugastoridae) from the Cordillera Real, Department La Paz, Bolivia. Zootaxa 2014, 3887, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Catenazzi, A. Phrynopus cophites. Reproduction. Herpetol. Rev. 2006, 37, 206. [Google Scholar]



| Taxon | 16S | 12S | COI | RAG1 | Tyr | Voucher Nbr | Reference |
|---|---|---|---|---|---|---|---|
| Barycholos pulcher | EU186709 | - | - | - | EU186765 | KU 217781 | [1] |
| Barycholos ternetzi | JX267466 | - | - | JX267543 | JX267680 | CFBH 19426 | [23] |
| Bryophryne bakersfield | KT276291 | KT276283 | - | - | - | MHNC 6007 | [12] |
| Bryophryne bakersfield | MF186344 | MF186287 | - | KT276278 | - | MHNC 6009 | [12] |
| Bryophryne bustamantei | MT437052 | - | - | MT431911 | - | MUSM 24537 | This study |
| Bryophryne bustamantei | CMT437053 | - | - | MT431912 | - | MUSM 24538 | This study |
| Bryophryne bustamantei | KT276293 | KT276286 | - | KT276280 | KT276296 | MHNC 6019 | [12] |
| Bryophryne cf. zonalis | MT437054 | - | MT435518 | - | - | CORBIDI 17475 | This study |
| Bryophryne cophites | EF493537 | - | - | EF493423 | EF493508 | KU173497 | [9] |
| Bryophryne cophites | KY652641 | - | KY672976 | KY672961 | KY681062 | AC 270.07 | [22] |
| Bryophryne hanssaueri | KY652642 | - | KY672977 | KY681084 | KY681063 | MUSM 27567 | [22] |
| Bryophryne nubilosus | KY652643 | - | KY672978 | KY681085 | KY681064 | MUSM 27882 | [22] |
| Bryophryne phuyuhampatu | MF419259 | - | - | - | - | CORBIDI 18224 | [16] |
| Bryophryne phuyuhampatu | MF419259 | - | - | - | - | MUBI 14654 | [16] |
| Bryophryne quellokunka | MT437061 | - | - | - | - | MUSM 27571 | This study |
| Bryophryne quellokunka | MF186387 | MF186309 | - | MF186526 | - | MNCN 43780 | [2] |
| Bryophryne sp. | MT437062 | - | - | MT431916 | - | MUSM 27961 | This study |
| Bryophryne sp. | MT437063 | - | - | MT431917 | - | AC 41.09 | This study |
| Bryophryne tocra | MF186396 | MF186315 | - | MF186541 | MF186583 | MNCN 43786 | [2] |
| Bryophryne wilakunka | MF186349 | MF186291 | - | - | - | MUBI 5425 | [2] |
| Bryophryne zonalis | MT437064 | - | - | - | - | MUSM 27939 | This study |
| Eleutherodactylus bilineatus | JX267324 | - | - | JX267556 | JX267691 | MNRJ 46476 | [23] |
| Euparkerella brasiliensis | JX267468 | - | - | JX267545 | JX267682 | - | [23] |
| Holoaden bradei | EF493366 | EF493378 | - | EF493449 | EU186779 | USNM 207945 | [9] |
| Holoaden luederwaldti | EU186710 | EU186728 | - | EU186747 | EU186768 | MZUSP 131872 | [1] |
| Holoaden luederwaldti | JX267470 | - | - | - | - | CFBH 19552 | [23] |
| Lynchius flavomaculatus | EU186667 | EU186667 | - | EU186745 | EU186766 | KU218210 | [1] |
| Lynchius nebulanastes | EU186704 | EU186704 | - | - | - | KU 181408 | [1] |
| Lynchius oblitus | KX470783 | KX470776 | - | KX470792 | KX470799 | MHNC 8614 | [25] |
| Lynchius parkeri | EU186705 | EU186705 | - | - | - | KU 181307 | [1] |
| Lynchius simmonsi | JF810004 | JF809940 | - | JF809915 | JF809894 | QZ 41639 | [26] |
| Microkayla adenopleura | MF186339 | - | - | - | - | MNCN 44809 | [2] |
| Microkayla adenopleura | MF186340 | MF186283 | - | MF186537 | MF186565 | MNCN 44810 | [2] |
| Microkayla ankohuma | - | MF186288 | - | - | - | MNKA 7280 | [2] |
| Microkayla ankohuma | - | MF186289 | - | - | - | CBF 5982 | [2] |
| Microkayla boettgeri | MF186352 | MF186293 | MF186456 | - | - | MNCN 43778 | [2] |
| Microkayla boettgeri | MF186353 | MF186294 | - | - | MF186559 | MUBI 5363 | [2] |
| Microkayla boettgeri | MF186354 | - | - | - | - | MUBI 5364 | [2] |
| Microkayla cf. iatamasi | MF186365 | - | - | - | - | MNCN-DNA 20927 | [2] |
| Microkayla chacaltaya | MF186357 | - | - | MF186532 | - | MNCN 42052 | [2] |
| Microkayla chapi | MF186417 | MF186328 | - | MF186540 | MF186562 | MNCN 43762 | [2] |
| Microkayla chilina | MF186411 | - | - | - | - | MUBI 5350 | [2] |
| Microkayla chilina | MF186414 | MF186327 | MF186457 | MF186539 | MF186561 | MNCN 43772 | [2] |
| Microkayla condoriri | MF186358 | - | - | - | - | CBF 5988 | [2] |
| Microkayla guillei | AY843720 | AY843720 | - | - | DQ282995 | AMNH A165108 | [9] |
| Microkayla iatamasi | AM039644 | AM039712 | - | - | - | MTD TD 1231 | [9] |
| Microkayla illampu | MF186373 | - | - | - | - | CBF 5999 | [2] |
| Microkayla kallawaya | MF186379 | - | - | - | - | MNCN 42509 | [2] |
| Microkayla katantika | MF186380 | - | MF186453 | - | - | CBF 6012 | [2] |
| Microkayla kempffi | MF186384 | - | - | - | - | MNCN 43646 | [2] |
| Microkayla quimsacruzis | MF186407 | - | - | - | - | MNCN 42039 | [2] |
| Microkayla saltator | AM039642 | AM039710 | - | - | - | MTD TD 1229 | [9] |
| Microkayla sp. Coscapa | MF186399 | - | - | - | - | CBF 6564 | [2] |
| Microkayla sp. Khatu River | MF186409 | - | - | - | - | MNCN 42034 | [2] |
| Microkayla teqta | MF186400 | MF186318 | - | - | MF186552 | MNCN 45702 | [2] |
| Microkayla utururo | MF186433 | - | - | - | - | MNCN 46987 | [2] |
| Microkayla wettsteini | MF186434 | MF186338 | - | MF186531 | MF186551 | CBF 6241 | [2] |
| Niceforonia brunnea | EF493357 | - | - | - | - | KU 178258 | [9] |
| Niceforonia dolops | EF493394 | - | - | - | - | - | [9] |
| Noblella heyeri | JX267541 | JX267463 | - | - | - | QCAZ 31471 | [23] |
| Noblella lochites | EU186699 | EU186699 | - | EU186756 | EU186777 | KU 177356 | [1] |
| Noblella losamigos | MN366392 | - | MN356099 | - | - | MVZ 292687 | [27] |
| Noblella losamigos | KY652644 | - | - | KY672962 | KY681065 | MUSA 6973 | [22] |
| Noblella losamigos | MN056358 | - | MN356098 | - | - | MUBI 17413 | [27] |
| Noblella madreselva | MN064565 | - | - | MN355547 | - | CORBIDI 15769 | [27] |
| Noblella myrmecoides | JX267542 | JX267464 | - | - | - | QCAZ 40180 | [23] |
| Noblella myrmecoides | MN056357 | - | - | - | - | CORBIDI PV45 | [28] |
| Noblella pygmaea | KY652645 | - | KY672979 | KY681086 | KY681066 | MUSM 24536 | [22] |
| Noblella sp. | AM039646 | AM039714 | - | - | - | MTD 45180 | [29] |
| Noblella sp. R | KY652646 | - | KY672980 | KY681087 | KY681067 | MUSM 27582 | [22] |
| Noblella thiuni | MK072732 | - | - | - | - | CORBIDI 18723 | [28] |
| Oreobates amarakaeri | JF809996 | JF809934 | - | JF809913 | JF809891 | MHNC 6975 | [26] |
| Oreobates ayacucho | JF809970 | JF809933 | - | JF809912 | JF809890 | MNCN IDlR5024 | [26] |
| Oreobates cruralis | EU186666 | EU186666 | - | EU186743 | EU186764 | KU 215462 | [1] |
| Oreobates gemcare | JF809960 | JF809930 | - | JF809909 | - | MHNC 6687 | [26] |
| Oreobates granulosus | EU368897 | JF809929 | - | JF809908 | JF809887 | MHNC 3396 | [30] |
| Phrynopus auriculatus | EF493708 | EF493708 | - | - | - | KU 291634 | [9] |
| Phrynopus barthlenae | AM039653 | AM039721 | - | - | - | SMF 81720 | [29] |
| Phrynopus bracki | EF493709 | EF493709 | - | EF493421 | - | USNM 286919 | [9] |
| Phrynopus bufoides | AM039645 | AM039713 | - | - | - | MHNSM 19860 | [29] |
| Phrynopus heimorum | AM039635 | AM039703 | MF186462 | MF186545 | MF186580 | MTD 45621 | [29] |
| Phrynopus horstpauli | AM039651 | AM039719 | - | - | - | MTD 44333 | [29] |
| Phrynopus inti | MF651902 | MF651909 | - | MF651917 | - | MUSM 31968 | [3] |
| Phrynopus kauneorum | AM039655 | AM039723 | - | - | - | MHNSM 20595 | [29] |
| Phrynopus peruanus | MG896582 | MG896605 | MG896615 | MG896626 | MG896631 | MUSM 38316 | [3] |
| Phrynopus pesantesi | AM039656 | AM039724 | - | - | - | MTD 45072 | [29] |
| Phrynopus spI | MG896589 | MG896606 | - | MG896629 | - | MUSM 33261 | [3] |
| Phrynopus tautzorum | AM039652 | AM039720 | - | - | - | MHNSM 20613 | [29] |
| Phrynopus tribulosus | EU186725 | EU186707 | - | - | - | KU 291630 | [1] |
| Pristimantis attenboroughi | KY594752 | - | KY962779 | KY962759 | - | MUSM 31186 | [10] |
| Pristimantis pluvialis | KX155577 | - | - | KY962769 | - | CORBIDI 11862 | [31] |
| Pristimantis reichlei | EF493707 | EF493707 | - | EF493436 | - | MHNSM 9267 | [9] |
| Pristimantis stictogaster | EF493704 | EF493704 | - | EF493445 | - | KU 291659 | [9] |
| Psychrophrynella chirihampatu | KU884559 | - | - | - | - | CORBIDI 16495 | [19] |
| Psychrophrynella chirihampatu | KU884560 | - | - | - | - | MHNC 14664 | [19] |
| Psychrophrynella glauca | MG837565 | - | - | - | - | CORBIDI 18729 | [20] |
| Psychrophrynella sp. | MT437065 | - | - | - | - | MUSM 27619 | This study |
| Psychrophrynella sp. | MT437066 | - | - | - | - | MTD 47488 | This study |
| Psychrophrynella sp. P | KY652660 | - | KY672992 | KY681089 | KY681081 | AC116.09 | [22] |
| Psychrophrynella sp. R | KY652661 | - | KY672993 | KY681090 | KY681082 | AC148.07 | [22] |
| Psychrophrynella usurpator | KY652662 | - | KY672994 | KY672975 | KY681083 | AC186.09 | [22] |
| Qosqophryne flammiventris | MT437055 | - | - | - | - | MTD 46890 | This study |
| Qosqophryne flammiventris | MT437056 | - | - | MT431913 | - | MUSM 27615 | This study |
| Qosqophryne gymnotis | MT437057 | - | - | MT431914 | - | MUSM 24546 | This study |
| Qosqophryne gymnotis | MT437058 | - | - | MT431915 | - | MUSM 24543 | This study |
| Qosqophryne mancoinca | MT437059 | - | MT435519 | - | - | MUBI 16068 | This study |
| Qosqophryne mancoinca | MT437060 | - | MT435520 | - | - | MUBI 16069 | This study |
| Locus | Primer | Sequence (5′-3′) | Reference | |
|---|---|---|---|---|
| 16S | 16SAR | F | CGCCTGTTTATCAAAAACAT | [33] |
| 16SBR | R | CCGGTCTGAACTCAGATCACGT | [33] | |
| 12S | L25195 | F | AAACTGGGATTAGATACCCCACTA | [33] |
| H2916 | R | GAGGGTGACGGGCGGTGTGT | [33] | |
| COI | dgLCO1490 | F | GGTCAACAAATCATAAAGAYATYGG | [34] |
| dgHCO2198 | R | TAAACTTCAGGGT GACCAAARAAYCA | [34] | |
| RAG1 | R182 | F | GCCATAACTGCTGGAGCATYAT | [9] |
| R270 | R | AGYAGATGTTGCCTGGGTCTTC | [9] | |
| Tyr | Tyr1C | F | GGCAGAGGAWCRTGCCAAGATGT | [35] |
| Tyr1G | R | TGCTGGGCRTCTCTCCARTCCCA | [35] |
| Characters | Q. gymnotis | Q. flammiventris | Q. mancoinca |
|---|---|---|---|
| Skin on dorsum | shagreen | Shagreen with small scattered tubercles | Shagreen with small conical tubercles |
| Skin on venter | smooth | Weakly areolate | smooth |
| Dorsolateral folds | Discontinuous, short | Discontinuous, short | Continuous, short |
| Tympanic membrane | + | + | + |
| Tympanic annulus | + | + | + |
| Dentigerous processes of vomers | + | ‒ | + |
| Vocal sac | + | + | + |
| Vocal slits | + | + | + |
| Nuptial pads | ‒ | ‒ | ‒ |
| Fingers with lateral fringes | + | ‒ | + |
| Toes with lateral fringes | + | ‒ | + |
| Inner tarsal fold | ‒ | ‒ | ‒ |
| Dorsum coloration | Reddish, grayish or purplish brown or dark gray with narrow tan middorsal stripe | Grayish brown | Reddish brown or grayish brown with narrow tan middorsal stripe |
| Venter coloration | Dark brown, tan, or reddish brown with pale gray flecks | Blackish brown with yellow, orange or pink blotches | Gray or pale bluish gray with reddish-brown reticulation |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catenazzi, A.; Mamani, L.; Lehr, E.; von May, R. A New Genus of Terrestrial-Breeding Frogs (Holoadeninae, Strabomantidae, Terrarana) from Southern Peru. Diversity 2020, 12, 184. https://doi.org/10.3390/d12050184
Catenazzi A, Mamani L, Lehr E, von May R. A New Genus of Terrestrial-Breeding Frogs (Holoadeninae, Strabomantidae, Terrarana) from Southern Peru. Diversity. 2020; 12(5):184. https://doi.org/10.3390/d12050184
Chicago/Turabian StyleCatenazzi, Alessandro, Luis Mamani, Edgar Lehr, and Rudolf von May. 2020. "A New Genus of Terrestrial-Breeding Frogs (Holoadeninae, Strabomantidae, Terrarana) from Southern Peru" Diversity 12, no. 5: 184. https://doi.org/10.3390/d12050184