4-(3-Phenyl-1H-pyrazolo[3,4-d]pyrimidin-1-yl)thieno[3,2-d]pyrimidine
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of 4-(3-Phenyl-1H-Pyrazolo[3,4-d]Pyrimidin-1-yl)Thieno[3,2-d]Pyrimidine 3
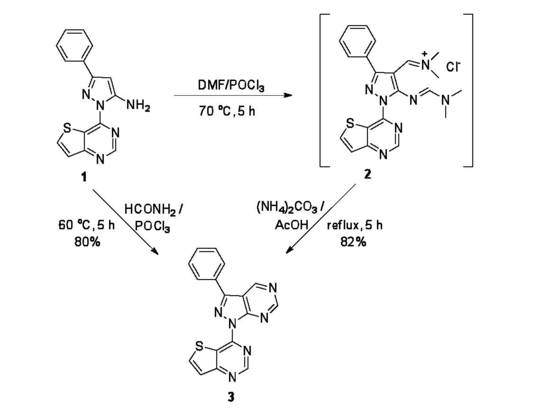
- (1)
- Method using Vilsmeier–Haack reagent: A solution of 3-phenyl-1-(thieno[3,2-d]pyrimidin-4-yl)-1H-pyrazol-5-amine 1 (100 mg, 0.34 mmol) and POCl3 (0.10 mL, 1.1 mmol, 3.0 equiv) in DMF (3 mL) was heated at 70 °C with stirring for 5 h. The mixture was cooled to room temperature and evaporated to dryness. Acetic acid (5 mL) and ammonium carbonate (105 mg, 1.1 mmol) were added to the residue, the reaction mixture was heated under reflux for 5 h. When the reaction was completed, the mixture was added to saturated aqueous sodium bicarbonate (15 mL) and extracted with CH2Cl2 (2 × 15 mL). The organic extracts were dried over MgSO4, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography (eluent: ethyl acetate/petroleum ether (bp 40–60 °C) = 1/10) to give the title compound 3 (92 mg, 82%) and recrystallized as light yellow needles, mp 263–264 °C (EtOH); TLC Rf = 0.25 (ethyl acetate/n-hexane = 20/80); 1H-NMR (400 MHz, CDCl3) δ 9.95 (s, 1H), 9.38 (s, 1H), 9.21 (s, 1H), 8.61 (d, J = 5.8 Hz, 1H), 8.36–8.34 (m, 2H), 7.74 (d, J = 5.8 Hz, 1H), 7.72–7.68 (m, 2H), 7.66–7.62 (m, 1H). 13C-NMR (100 MHz, CDCl3) 163.3, 157.0, 154.8, 154.2, 153.6, 152.2, 146.4, 141.4, 130.5, 130.0, 129.4 (2 × C), 127.5 (2 × C), 124.1, 118.5, 114.6. IR (KBr) 1620 (C = N), 755, 690 cm−1. UV (λmax in MeOH, log ε) 350 (4.15), 288 (3.90) nm. MS (APCI) m/z = 331.21 (MH+, 96%). Anal. calcd. for C17H10N6S, %: C, 61.80; H, 3.05; N, 25.44. Found, %: C, 61.95; H, 3.10; N, 25.51.
- (2)
- Direct method using a modified Vilsmeier–Haack reagent: A solution of 3-phenyl-1-(thieno[3,2-d]pyrimidin-4-yl)-1H-pyrazol-5-amine 1 (100 mg, 0.34 mmol) and POCl3 (0.10 mL, 1.1 mmol, 3.0 equiv) in formamide (3 mL) was heated at 60 °C with stirring for 5 h. After the completion, the reaction mixture was concentrated and worked-up in the same way as above. The residue was purified by column chromatography (eluent: ethyl acetate/petroleum ether (bp 40–60 °C) = 1/10) to give the title compound 3 (89 mg, 80%).
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ali, E.M.H.; Abdel-Maksoud, M.S.; Oh, C.-H. Thieno[2,3-d]pyrimidines as a promising scaffold in medicinal chemistry: Recent advances. Bioorg. Med. Chem. 2019, 27, 1159–1194. [Google Scholar] [CrossRef] [PubMed]
- Ghith, A.; Ismail, N.S.M.; Youssef, K.; Abouzid, K.A.M. Medicinal attributes of thienopyrimidine based scaffold targeting tyrosine kinases and their potential anticancer activities. Arch. Pharm. 2017, 350, 1700242. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, K.R.A.; Bakr, R.B. New advances in synthesis and clinical aspects of pyrazolo[3,4-d]pyrimidine scaffolds. Bioorg. Chem. 2018, 78, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.; Kumar, R. Medical attributes of pyrazolo[3,4-d]pyrimidines: A review. Bioorg. Med. Chem. Lett. 2013, 21, 5657–5668. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, R.; Prencipe, F.; Oliva, P.; Baraldi, S.; Baraldi, P.G.; Schiaffino Ortega, S.; Chayah, M.; Salvador, M.K.; Lopez-Cara, L.C.; Brancale, A.; et al. Design, synthesis, and biological evaluation of 6-substituted thieno[3,2-d]pyrimidine analogues as dual epidermal growth factor receptor kinase and microtubule inhibitors. J. Med. Chem. 2019, 2, 1274–1290. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Dong, Y.; Li, M.; Wang, R.; Zhang, X.; Gong, P.; Zhao, Y. Design, synthesis and biological evaluation of novel thieno[3,2-d]pyrimidine and quinazoline derivatives as potent antitumor agents. Bioorg. Chem. 2019, 90, 103086. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.N.; El-Gazzar, A.-R.B.A.; Zaki, M.E.A. Simple approach to thieno[3,2-d]pyrimidines as new scaffolds of antimicrobial activities. Acta Pharm. 2016, 66, 331–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Kang, D.; Chen, M.; Wu, G.; Feng, D.; Zhao, T.; Zhou, Z.; Huo, Z.; Jing, L.; Zuo, X.; et al. Design, synthesis, and antiviral evaluation of novel hydrazone-substituted thiophene[3,2-d]pyrimidine derivatives as potent human immunodeficiency virus-1 inhibitors. Chem. Biol. Drug Des. 2018, 92, 2009–2021. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; Graziano, A.C.E.; Marrazzo, A.; Gemmellaro, P.; Santagati, A.; Cardile, V. Synthesis and biological evaluation of new benzo-thieno[3,2-d]pyrimidin-4-one sulphonamide thio-derivatives as potent selective cyclooxygenase-2 inhibitors. Mol. Divers. 2013, 17, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Xu, F.-Z.; Zhu, Y.-Y.; Song, B.; Luo, D.; Yu, G.; Chen, S.; Xue, W.; Wu, J. Pyrazolo[3,4-d]pyrimidine derivatives containing a Schiff base moiety as potential antiviral agents. Bioorg. Med. Chem. Lett. 2018, 28, 2979–2984. [Google Scholar] [CrossRef] [PubMed]
- Cherukupalli, S.; Chandrasekaren, B.; Krystof, V.; Aleti, R.R.; Sayyad, N.; Merugu, S.R.; Kushwaha, N.D.; Karpoormath, R. Synthesis, anticancer evaluation, and molecular docking studies of some novel 4,6-disubstituted pyrazolo[3,4-d]pyrimidines as cyclin-dependent kinase 2 (CDK2) inhibitors. Bioorg. Chem. 2018, 79, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Tageldin, G.N.; Fahmy, S.M.; Ashour, H.M.; Khalil, M.A.; Nassra, R.A.; Labouta, I.M. Design, synthesis and evaluation of some pyrazolo[3,4-d]pyrimidines as anti-inflammatory agents. Bioorg. Chem. 2018, 78, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, L.M.; da Silva, E.R.; Hoelz, L.V.B.; Souza, D.L.; Come, J.A.A.S.S.; Cardoso-Santos, C.; Batista, M.M.; Soeiro, M.N.C.; Boechat, N.; Pinheiro, L.C.S. New pyrazolopyrimidine derivatives as Leishmania amazonensis arginase inhibitors. Bioorg. Med. Chem. 2019, 27, 3061–3069. [Google Scholar] [CrossRef] [PubMed]
- Hassaneen, H.D.; Saleh, F.M.; Abdallah, T.A.; Mohamed, M.F.; Mohamed, Y.S.; Awad, E.M.; Abdelhamid, I.A. Synthesis, cytotoxicity, antimicrobial and docking simulation of novel pyrazolo[3,4-d]pyrimidine and pyrazolo[4,3-e]triazolo[3,4-c]pyrimidine derivatives. Mini Rev. Med. Chem. 2019, 19, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Song, Y.-H. Synthesis of thienopyrimidine-pyrazolo[3,4-b]pyridine hybrids. Heterocycl. Commun. 2017, 23, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.-J.; Kim, S.M.; Rho, M.-C.; Lee, S.W.; Song, Y.-H. Synthesis of thienopyrimidine derivatives as inhibitors of STAT3 activation induced by IL-6. J. Microbiol. Biotechnol. 2019, 29, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Simay, A.; Takacs, K.; Horvath, K.; Dvortsak, P. Vilsmeier-Haack reaction of 5-amino- and 5-acylaminopyrazoles. Acta Chim. Acad. Sci. Hung. 1980, 105, 127–140. [Google Scholar]
- Chang, C.-H.; Tsai, H.J.; Huang, Y.-Y.; Lin, H.-Y.; Wang, L.Y.; Wu, T.S.; Wong, F.F. Selective synthesis of pyrazolo[3,4-d]pyrimidine, N-(1H-pyrazol-5-yl)formamide, or N-(1H-pyrazol-5-yl)formamidine derivatives from N-1-substituted-5-aminopyrazoles with new Vilsmeier-type reagents. Tetrahedron 2013, 69, 1378–1386. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, W.; Li, Y. Discovery of novel inhibitors of signal transducer and activator of transcription 3 (STAT3) signaling pathway by virtual screening. Eur. J. Med. Chem. 2013, 62, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-C.; Zhang, G.-Y. Inhibitory effect of genistein on activation of STAT3 induced by brain ischemia/reperfusion in rat hippocampus. Acta Pharmacol. Sin. 2003, 24, 1131–1136. [Google Scholar] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, E.S.; Kim, S.M.; Song, Y.-H. 4-(3-Phenyl-1H-pyrazolo[3,4-d]pyrimidin-1-yl)thieno[3,2-d]pyrimidine. Molbank 2020, 2020, M1136. https://doi.org/10.3390/M1136
Noh ES, Kim SM, Song Y-H. 4-(3-Phenyl-1H-pyrazolo[3,4-d]pyrimidin-1-yl)thieno[3,2-d]pyrimidine. Molbank. 2020; 2020(2):M1136. https://doi.org/10.3390/M1136
Chicago/Turabian StyleNoh, Eun Sun, Sung Min Kim, and Yang-Heon Song. 2020. "4-(3-Phenyl-1H-pyrazolo[3,4-d]pyrimidin-1-yl)thieno[3,2-d]pyrimidine" Molbank 2020, no. 2: M1136. https://doi.org/10.3390/M1136