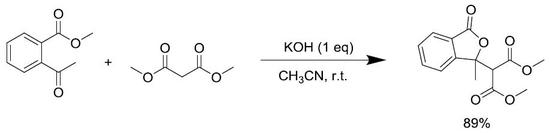
Dimethyl 2-(1-Methyl-3-oxo-1,3-dihydroisobenzofuran-1-yl)malonate
Abstract
:1. Introduction
2. Result and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for Synthesis and Characterization of 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karmakar, R.; Pahari, P.; Mal, D. Phthalides and Phthalans: Synthetic Methodologies and Their Applications in the Total Synthesis. Chem. Rev. 2014, 114, 6213–6284. [Google Scholar] [CrossRef] [PubMed]
- Bella, M.; Gasperi, T. Organocatalytic Formation of Quaternary Stereocenters. Synthesis 2009, 10, 1583–1614. [Google Scholar] [CrossRef]
- Li, G.; Yin, D.; Liang, X.T. A Facile Synthesis of 3-Substituted Phthalides. Synth. Commun. 2004, 34, 1183–1189. [Google Scholar] [CrossRef]
- Di Mola, A.; Croce, G.; More, V.; De Caprariis, P.; Filosa, R.; Massa, A. Active methylene compounds in a very effective approach to 3-substituted isobenzofuranones through tandem aldol/lactonization reactions. Tetrahedron 2012, 68, 6146–6151. [Google Scholar] [CrossRef]
- More, V.; Di Mola, A.; Perillo, M.; De Caprariis, P.; Filosa, R.; Peduto, A.; Massa, A. The aldol addition of readily enolizable 1,3-dicarbonyl compounds to 2-cyanobenzaldehyde in the synthesis of 3-substituted isoindolinones. Synthesis 2011, 18, 3027–3031. [Google Scholar]
- Di Mola, A.; Di Martino, M.; Capaccio, V.; Pierri, G.; Palombi, L.; Tedesco, C.; Massa, A. Synthesis of 2-Acetylbenzonitriles and Their Reactivity in Tandem Reactions with Carbon and Hetero Nucleophiles: Easy Access to 3,3-Disubstituted Isoindolinones. Eur. J. Org. Chem. 2018, 1699–1708. [Google Scholar] [CrossRef]
- Di Mola, A.; Macchia, A.; Tedesco, C.; Pierri, G.; Palombi, L.; Filosa, R.; Massa, A. Synthetic Strategies and Cascade Reactions of 2-Cyanobenzophenones for the Access to Diverse 3,3-Disubstituted Isoindolinones and 3-Aryl-3-Hydroxyisoindolinones. Chem. Select. 2019, 4, 4820–4826. [Google Scholar] [CrossRef]


| Entry | Base (eq) | Solvent (M) | T (°C) | t (h) | Yield (%) a |
|---|---|---|---|---|---|
| 1 | K2CO3 (1) | Solvent-free | t.a. | 15 | n.d. |
| 2 | K2CO3 (2) | CH3CN (1.40) | t.a. | 15 | n.d. |
| 3 | K2CO3 (3) | CH3CN (1.87) | 50 | 24 | 46 |
| 4 | K2CO3 (3) | CH3CN (1.87) | 50 | 48 | 58 |
| 5 | KOH (1) | CH3CN (1.87) | t.a. | 24 | 68 |
| 6 | KOH (1) | CH3CN (1.87) | t.a. | 48 | 89 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mola, A.D.; Filosa, R.; Massa, A. Dimethyl 2-(1-Methyl-3-oxo-1,3-dihydroisobenzofuran-1-yl)malonate. Molbank 2020, 2020, M1124. https://doi.org/10.3390/M1124
Mola AD, Filosa R, Massa A. Dimethyl 2-(1-Methyl-3-oxo-1,3-dihydroisobenzofuran-1-yl)malonate. Molbank. 2020; 2020(2):M1124. https://doi.org/10.3390/M1124
Chicago/Turabian StyleMola, Antonia Di, Rosanna Filosa, and Antonio Massa. 2020. "Dimethyl 2-(1-Methyl-3-oxo-1,3-dihydroisobenzofuran-1-yl)malonate" Molbank 2020, no. 2: M1124. https://doi.org/10.3390/M1124