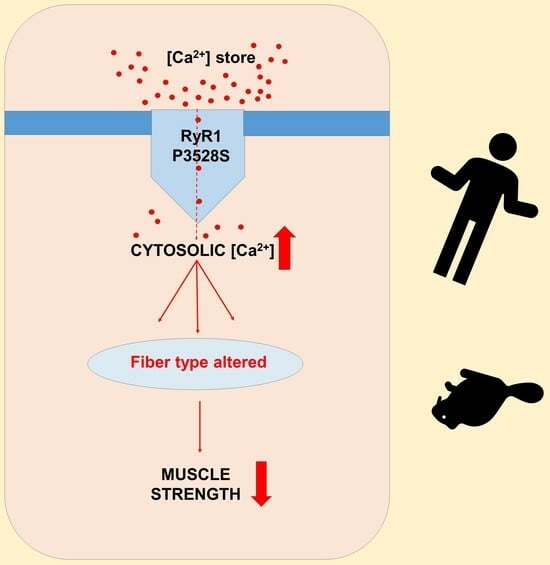
The RyR1 P3528S Substitution Alters Mouse Skeletal Muscle Contractile Properties and RyR1 Ion Channel Gating
Abstract
:1. Introduction
2. Results
2.1. Mouse Appearance and Weight
| Young Males Weight (g) | Young Females Weight (g) | Old Males Weight (g) | Old Females Weight (g) | |
|---|---|---|---|---|
| WT/WT | 24.3 ± 0.8 | 20.5 ± 0.9 @ | 33.0 ± 1.7 * | 28.9 ± 1.2 *@ |
| (N = 8) | (N = 8) | (N = 5) | (N = 7) | |
| WT/MUT | 25.0 ± 0.9 | 18.5 ± 0.7 @ | 33.7 ± 1.0 * | 30.6 ± 1.0 *@ |
| (N = 6) | (N = 5) | (N = 15) | (N = 13) | |
| MUT/MUT | 26.7 ± 1.3 | 21.4 ± 0.8 @ | 33.7 ± 1.6 * | 27.6 ± 1.4 *@ |
| (N = 3) | (N = 6) | (N = 7) | (N = 10) |

2.2. Mouse Mobility and Strength
2.3. Contractile Properties of Isolated EDL and SOL Muscles
2.4. Mechanically Skinned Fibres
2.5. RyR1 Single Channel Activity
2.5.1. Effects of the P3528S Mutation on Channel Characteristics
2.5.2. An Age-Dependent Increase in Activity
2.6. Histological Assessment
2.7. Myosin Heavy Chain (MyHC) Composition
3. Discussion
3.1. Elevated Cytoplasmic [Ca2+] Could Alter Expression of Factors Determining Fibre Type
3.2. Potential Consequences of Increased RyR1 Activity with 1 µM Cytoplasmic Ca2+ on Ca2+ Homeostasis
3.3. Fibre Type Changes Indicated in Mechanically Skinned Fibres and in Fibre Cross-Sectional Areas
3.4. The P3538S Substitution Could Alter Mouse Behaviour
3.5. Possible Effects of the P3528S Substitution on RyR1 Structure and Ion Channel Gating
3.6. The P3528S Substitution on One Allele Is Sufficient to Alter Function
3.7. Embryonic Importance of RyR1
3.8. Possible Limiting Factors
4. Material and Methods
4.1. Generation and Genotyping of the RyR1-P3 Mice
4.2. Animal Experimentation
Preliminary Phenotype Assessment
- Ambulation. The ambulation rate of mice during free exploration of a 52 × 31 cm container was assessed. The animal was placed in a cage and allowed free movement. The distance that the mouse covered was measured from a video recorded over a 3 min period following a ~5 min settling period [42]. Ambulation rate is expressed as m/min.
- Strength tests. Two strength tests were used [43,44]. In the first test, the mice were placed onto a wire screen that was then inverted and held ~30 cm above a soft surface for a maximum period of 5.5 min. The mice clung to the screen with their forelimbs before either dropping onto the soft surface or being lowered onto the surface if they maintained their grip for 5.5 min. The time that the mice held onto the screen (“hold duration”) was recorded. In many cases, mice held onto the screen for the 5.5 min period with the “hold duration” achieving a “glass ceiling” of 5.5 min. In the second test, the ability of mice to hold weights constructed of chains with increasing numbers of rings, for period of up to 3 s, was examined. The minimum weight was 18 g (2 rings) and the heaviest was 45 g (5 rings). Each set of rings was attached to fine wire tangles that the mouse grabbed with its front paws when held above the tangles. The mouse was presented with the lightest chain first; if that chain was held for 3 s, the animal was rested for ~1 min and then presented with next heaviest chain and so on up to the heaviest chain. Generally, mice were able to hold between 27 and 36 g for 3 s without dropping the chain. A “hold score” was calculated based on the chain weight and the length of time that the weight was held. Weight #1 (2 rings) was assigned a value of 1; weight #2 (3 rings) a value of 2; weight #3 (4 rings) a value of 3; and weight #4 (5 rings) a value of 4. Therefore, for example, if weights #1 and #2 were held for 3 s and weight #3 for 1 s, the score in arbitrary units (AU) was calculated as (1 × 3) + (2 × 3) + (3 × 1), giving a score of 12 AU.
4.3. Euthanasia and Tissue Collection
4.3.1. Whole Muscle Experiments
4.3.2. Single-Fibre Preparation for Skinned Fibre Experiments
4.3.3. Solutions for Mechanically Skinned Fibre Experiments
4.3.4. Contractile Apparatus Measurements of Skinned Fibres
4.4. Single-Channel Electrophysiology
4.4.1. Vesicle Preparation
4.4.2. Lipid Bilayer Formation and Vesicle Incorporation
4.4.3. Analysis of RyR1 Channel Activity
4.5. Histology and Myosin Heavy Chain Analysis
4.5.1. Histology
4.5.2. Myosin Heavy Chain Analysis
- Tissue Preparation. The procedure was modified from Talmadge and Roy (1993) [49]. Frozen limb muscle from two mice of a similar ages and genotype were combined for each preparation or 100 mg of frozen rat EDL and SOL muscles was used. The tissue was thawed in 2 mL Eppendorf tubes and covered in sufficient Myosin Heavy Chain Homogenizing Buffer (MyHC-HB) containing (in mM) 250 sucrose, 100 KCl, 5 EDTA, 40 DTT and 20 Tris-HCL, pH 6.8. Protease inhibitors were added containing (in mM) 1 benzamadine, 0.5 PMSF, 3000 anti-calpain I, 3000 anti-calpain II, 2300 leupeptin and 1.460 pepstatin A. The tissue was homogenized 3 times for 5 s each, and then centrifuged at 13,500× g for 20 min at 4 °C in an Eppendorf 5425R centrifuge. The pellet was resuspended and homogenized in 600–800 µL of MyHC-HB using a Potter homogenizer. The protein concentration of the pellet suspension was determined using the Bicinchoninic Acid Assay (BCA) and adjusted to 0.6 mg/mL for mouse muscles or 0.3 mg/mL for rat muscles and then stored at −80 °C.
- MyHC SDS-PAGE. The methods were based on Mizunoya et al. (2008) [50], with minor modifications to improve separation of proteins with molecular weights between 150 and 250 kDa. The running gel (8% w/v acrylamide-bis acrylamide (99:1), 35% v/v glycerol, 0.2 M Tris-HCl (ph-8.8), 0.1 M glycine, 0.4% w/v SDS, 0.1% w/v ammonium persulfate (APS), 0.05% v/v N,N,N′,N′-tetramethylethylenediamine (TEMED)) was polymerized for 1 h at room temperature. The stacking gel (4% w/v acrylamide-bis acrylamide (49:1), 30% v/v glycerol, 0.07 M Tris-HCl (ph-6.7), 4 mM EDTA, 0.4% w/v SDS, 0.1% w/v ammonium persulfate (APS), 0.05% v/v TEMED) was polymerized for 1 h at room temperature, and then overnight at 4° C. The wells were loaded with 20 µL of the muscle samples and run at a constant voltage of 70 V for 24 h in a cold room (~4° C) with constant stirring. The lower running buffer contained (in mM) 50 Tris, 75 glycine and 0.05% w/v SDS. The upper running buffer contained 300 mM Tris, 450 mM glycine, 0.3% w/v SDS and 0.12% v/v β-ME. After electrophoresis, the gels were either silver-stained (Bio-Rad, Cat. No. 1610449) or transferred onto polyvinylidene difluoride (PVDF) membranes for immunoblotting.
- Western blots. MyHC gels were equilibrated in transfer buffer (37 mM Tris, 140 mM glycine, 20% methanol) for 30 min and then transferred onto PVDF membranes using a wet transfer system under the following conditions: constant voltage of 100 V for 60 min followed by 30 min starting at 0.5A. Membranes were blocked in 5% skim milk in phosphate-buffered saline with 0.05% Tween 20 (PBST) for 1 h and subsequently incubated in the primary antibody overnight at 4° C. The membranes were then washed several times in PBST and incubated in the HRP-conjugated secondary antibody for 2 h at room temperature. They were then washed several times in PBST and imaged in the Bio-Rad ChemiDoc system after exposure to the ECL substrate (Thermo Fisher SuperSignal West Pico PLUS Chemiluminescent Substrate, Cat. No. 34577). Bands corresponding to type I, type IIB, type IIA and type IIX myosin isoforms were identified by their molecular masses relative to type IIB, which was confirmed by Western Blot, using the primary Myosin IIb Antibody#3404 (Cell Signaling Technology, Danvers, MA, USA). The secondary antibody was goat α-rabbit S-2004 obtained from Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA.
4.6. Statistics
4.6.1. Phenotype Characteristics, Lipid Bilayer Electrophysiology and Histology
4.6.2. Muscle Contractility
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Witherspoon, J.W.; Meilleur, K. Review of RyR1 pathway and associated pathomechanisms. Acta Neuropathol. Commun. 2016, 4, 121. [Google Scholar] [CrossRef] [PubMed]
- Romero, N.B. Centronuclear myopathies: A widening concept. Neuromuscul. Disord. 2010, 20, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Rokach, O.; Feng, L.; Munteanu, I.; Mamchaoui, K.; Wilmshurst, J.M.; Sewry, C.; Manzur, A.Y.; Pillay, K.; Mouly, V.; et al. RyR1 deficiency in congenital myopathies disrupts excitation-contraction coupling. Hum. Mutat. 2013, 34, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Jungbluth, H.; Treves, S.; Zorzato, F.; Sarkozy, A.; Ochala, J.; Sewry, C.; Phadke, R.; Gautel, M.; Muntoni, F. Congenital myopathies: Disorders of excitation-contraction coupling and muscle contraction. Nat. Rev. Neurol. 2018, 14, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.; Garcia-Castañeda, M.; Michelucci, A.; Sabha, N.; Malik, S.; Groom, L.; LaPierre, L.W.; Dowling, J.J.; Dirksen, R.T. Mouse model of severe recessive RYR1-related myopathy. Hum. Mol. Genet. 2019, 28, 3024–3036. [Google Scholar] [CrossRef] [PubMed]
- Amburgey, K.; Garcia-Castañeda, M.; Michelucci, A.; Sabha, N.; Malik, S.; Groom, L.; LaPierre, L.W.; Dowling, J.J.; Dirksen, R.T. Genotype-phenotype correlations in recessive RYR1-related myopathies. Orphanet J. Rare Dis. 2013, 8, 117. [Google Scholar] [CrossRef]
- Klein, A.; Lillis, S.; Munteanu, I.; Scoto, M.; Zhou, H.; Quinlivan, R.; Straub, V.; Manzur, A.Y.; Roper, H.; Jeannet, P.-Y.; et al. Clinical and genetic findings in a large cohort of patients with ryanodine receptor 1 gene-associated myopathies. Hum. Mutat. 2012, 33, 981–988. [Google Scholar] [CrossRef]
- Ferreiro, A.; Monnier, N.; Romero, N.B.; Leroy, J.; Bönnemann, C.; Haenggeli, C.; Straub, V.; Voss, W.D.; Nivoche, Y.; Jungbluth, H.; et al. A recessive form of central core disease, transiently presenting as multi-minicore disease, is associated with a homozygous mutation in the ryanodine receptor type 1 gene. Ann. Neurol. 2002, 51, 750–759. [Google Scholar] [CrossRef]
- Ducreux, S.; Zorzato, F.; Ferreiro, A.; Jungbluth, H.; Muntoni, F.; Monnier, N.; Müller, C.R.; Treves, S. Functional properties of ryanodine receptors carrying three amino acid substitutions identified in patients affected by multi-minicore disease and central core disease, expressed in immortalized lymphocytes. Biochem. J. 2006, 395, 259–266. [Google Scholar] [CrossRef]
- Zhang, K.M.; Wright, L.D.; Hu, P.; Spratt, J.A.; Wechsler, A.S.; Briggs, F.N. Induction of molecular and mechanical transformations in canine skeletal muscle by chronic neuromuscular stimulation. J. Muscle Res. Cell Motil. 1997, 18, 81–90. [Google Scholar] [CrossRef]
- Parry, D.J.; DiCori, S. The relationship between post-tetanic potentiation of motor units and myosin isoforms in mouse soleus muscle. Can. J. Physiol. Pharmacol. 1990, 68, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Schwaller, B.; Dick, J.; Dhoot, G.; Carroll, S.; Vrbova, G.; Nicotera, P.; Pette, D.; Wyss, A.; Bluethmann, H.; Hunziker, W.; et al. Prolonged contraction-relaxation cycle of fast-twitch muscles in parvalbumin knockout mice. Am. J. Physiol. 1999, 276, C395–C403. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [PubMed]
- Bortolotto, S.K.; Cellini, M.; Stephenson, D.G.; Stephenson, G.M. MHC isoform composition and Ca(2+)- or Sr(2+)-activation properties of rat skeletal muscle fibers. Am. J. Physiol. Cell Physiol. 2000, 279, C1564–C1577. [Google Scholar] [CrossRef] [PubMed]
- Lynch, G.S.; Stephenson, D.G.; Williams, D.A. Analysis of Ca2+ and Sr2+ activation characteristics in skinned muscle fibre preparations with different proportions of myofibrillar isoforms. J. Muscle Res. Cell Motil. 1995, 16, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Trinh, H.H.; Lamb, G.D. Matching of sarcoplasmic reticulum and contractile properties in rat fast- and slow-twitch muscle fibres. Clin. Exp. Pharmacol. Physiol. 2006, 33, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Copello, J.A.; Barg, S.; Onoue, H.; Fleischer, S. Heterogeneity of Ca2+ gating of skeletal muscle and cardiac ryanodine receptors. Biophys. J. 1997, 73, 141–156. [Google Scholar] [CrossRef]
- Salvage, S.C.; Gallant, E.M.; Fraser, J.A.; Huang CL, H.; Dulhunty, A.F. Flecainide Paradoxically Activates Cardiac Ryanodine Receptor Channels under Low Activity Conditions: A Potential Pro-Arrhythmic Action. Cells 2021, 10, 2101. [Google Scholar] [CrossRef]
- Haarmann, C.S.; Green, D.; Casarotto, M.G.; Laver, D.R.; Dulhunty, A.F. The random-coil ‘C’ fragment of the dihydropyridine receptor II-III loop can activate or inhibit native skeletal ryanodine receptors. Biochem. J. 2003, 372 Pt 2, 305–316. [Google Scholar] [CrossRef]
- Dulhunty, A.F.; Curtis, S.M.; Watson, S.; Cengia, L.; Casarotto, M.G. Multiple actions of imperatoxin A on ryanodine receptors: Interactions with the II-III loop “A” fragment. J. Biol. Chem. 2004, 279, 11853–11862. [Google Scholar] [CrossRef]
- Richardson, S.J.; Thekkedam, C.G.; Casarotto, M.G.; Beard, N.A.; Dulhunty, A.F. FKBP12 binds to the cardiac ryanodine receptor with negative cooperativity: Implications for heart muscle physiology in health and disease. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20220169. [Google Scholar] [CrossRef] [PubMed]
- Augusto, V.P.; Padovani, C.R.; Campos, G.E.R. Skeletal muscle fiber types in C57BL6J mice. Braz. J. Morphol. Sci. 2004, 21, 89–94. [Google Scholar]
- Sreter, F.A.; Lopez, J.R.; Alamo, L.; Mabuchi, K.; Gergely, J. Changes in intracellular ionized Ca concentration associated with muscle fiber type transformation. Am. J. Physiol. 1987, 253 Pt 1, C296–C300. [Google Scholar] [CrossRef] [PubMed]
- Kubis, H.P.; Haller, E.-A.; Wetzel, P.; Gros, G. Adult fast myosin pattern and Ca2+-induced slow myosin pattern in primary skeletal muscle culture. Proc. Natl. Acad. Sci. USA 1997, 94, 4205–4210. [Google Scholar] [CrossRef] [PubMed]
- Pette, D.; Staron, R.S. Mammalian skeletal muscle fiber type transitions. Int. Rev. Cytol. 1997, 170, 143–223. [Google Scholar] [PubMed]
- De Crescenzo, V.; Fogarty, K.E.; Lefkowitz, J.J.; Bellve, K.D.; Zvaritch, E.; MacLennan, D.H.; Walsh, J.V., Jr. Type 1 ryanodine receptor knock-in mutation causing central core disease of skeletal muscle also displays a neuronal phenotype. Proc. Natl. Acad. Sci. USA 2012, 109, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Filipova, D.; Walter, A.M.; Gaspar, J.A.; Brunn, A.; Linde, N.F.; Ardestani, M.A.; Deckert, M.; Hescheler, J.; Pfitzer, G.; Sachinidis, A.; et al. Gene profiling of embryonic skeletal muscle lacking type I ryanodine receptor Ca(2+) release channel. Sci. Rep. 2016, 6, 20050. [Google Scholar] [CrossRef]
- Salvage, S.C.; Huang CL, H.; Fraser, J.A.; Dulhunty, A.F. How does flecainide impact RyR2 channel function? J. Gen. Physiol. 2022, 154, e202213089. [Google Scholar] [CrossRef]
- Furuichi, T.; Furutama, D.; Hakamata, Y.; Nakai, J.; Takeshima, H.; Mikoshiba, K. Multiple types of ryanodine receptor/Ca2+ release channels are differentially expressed in rabbit brain. J. Neurosci. 1994, 14, 4794–4805. [Google Scholar] [CrossRef]
- Nagrani, T.; Siyamwala, M.; Vahid, G.; Bekheit, S. Ryanodine calcium channel: A novel channelopathy for seizures. Neurologist 2011, 17, 91–94. [Google Scholar] [CrossRef]
- Takano, K.; Liu, D.; Tarpey, P.; Gallant, E.; Lam, A.; Witham, S.; Alexov, E.; Chaubey, A.; Stevenson, R.E.; Schwartz, C.E.; et al. An X-linked channelopathy with cardiomegaly due to a CLIC2 mutation enhancing ryanodine receptor channel activity. Hum. Mol. Genet. 2012, 21, 4497–4507. [Google Scholar] [CrossRef] [PubMed]
- Samso, M. A guide to the 3D structure of the ryanodine receptor type 1 by cryoEM. Protein Sci. 2017, 26, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Steele, T.W.E.; Samso, M. The FKBP12 subunit modifies the long-range allosterism of the ryanodine receptor. J. Struct. Biol. 2019, 205, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Woll, K.A.; Haji-Ghassemi, O.; Van Petegem, F. Pathological conformations of disease mutant Ryanodine Receptors revealed by cryo-EM. Nat. Commun. 2021, 12, 807. [Google Scholar] [CrossRef] [PubMed]
- Iyer, K.A.; Hu, Y.; Nayak, A.R.; Kurebayashi, N.; Murayama, T.; Samsó, M. Structural mechanism of two gain-of-function cardiac and skeletal RyR mutations at an equivalent site by cryo-EM. Sci. Adv. 2020, 6, eabb2964. [Google Scholar] [CrossRef] [PubMed]
- Bharucha-Goebel, D.X.; Santi, M.; Medne, L.; Zukosky, K.; Dastgir, J.; Shieh, P.B.; Winder, T.; Tennekoon, G.; Finkel, R.S.; Dowling, J.J.; et al. Severe congenital RYR1-associated myopathy: The expanding clinicopathologic and genetic spectrum. Neurology 2013, 80, 1584–1589. [Google Scholar] [CrossRef]
- Gillard, E.F.; Otsu, K.; Fujii, J.; Khanna, V.K.; de Leon, S.; Derdemezi, J.; Britt, B.A.; Duff, C.L.; Worton, R.G.; MacLennan, D.H. A substitution of cysteine for arginine 614 in the ryanodine receptor is potentially causative of human malignant hyperthermia. Genomics 1991, 11, 751–755. [Google Scholar] [CrossRef]
- Lyfenko, A.D.; Ducreux, S.; Wang, Y.; Xu, L.; Zorzato, F.; Ferreiro, A.; Meissner, G.; Treves, S.; Dirksen, R.T. Two central core disease (CCD) deletions in the C-terminal region of RYR1 alter muscle excitation-contraction (EC) coupling by distinct mechanisms. Hum. Mutat. 2007, 28, 61–68. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, J.; Zhao, Z.; Shen, H.; Bing, Q.; Li, N. Next generation sequencing reveals ryanodine receptor 1 mutations in a Chinese central core disease cohort. Muscle Nerve 2016, 54, 432–438. [Google Scholar] [CrossRef]
- Gurumurthy, C.B.; O’brien, A.R.; Quadros, R.M.; Adams, J.; Alcaide, P.; Ayabe, S.; Ballard, J.; Batra, S.K.; Beauchamp, M.-C.; Becker, K.A.; et al. Reproducibility of CRISPR-Cas9 methods for generation of conditional mouse alleles: A multi-center evaluation. Genome Biol. 2019, 20, 171. [Google Scholar] [CrossRef]
- Ostermeier, G.C.; Wiles, M.V.; Farley, J.S.; Taft, R.A. Conserving, distributing and managing genetically modified mouse lines by sperm cryopreservation. PLoS ONE 2008, 3, e2792. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, E.M.; Crosbie-Watson, R.H. A Simple and Low-cost Assay for Measuring Ambulation in Mouse Models of Muscular Dystrophy. J. Vis. Exp. 2017, 130, e56772. [Google Scholar]
- Deacon, R.M. Measuring the strength of mice. J. Vis. Exp. 2013, 76, e2610. [Google Scholar]
- Hoffman, E.; Winder, S.J. A Modified Wire Hanging Apparatus for Small Animal Muscle Function Testing. PLoS Curr. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Stupka, N.; Gregorevic, P.; Plant, D.R.; Lynch, G.S. The calcineurin signal transduction pathway is essential for successful muscle regeneration in mdx dystrophic mice. Acta Neuropathol. 2004, 107, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Lynch, G.S.; Hinkle, R.T.; Chamberlain, J.S.; Brooks, S.V.; Faulkner, J.A. Force and power output of fast and slow skeletal muscles from mdx mice 6–28 months old. J. Physiol. 2001, 535 Pt 2, 591–600. [Google Scholar] [CrossRef]
- Dutka, T.L.; Lamboley, C.R.; McKenna, M.J.; Murphy, R.M.; Lamb, G.D. Effects of carnosine on contractile apparatus Ca2+ sensitivity and sarcoplasmic reticulum Ca2+ release in human skeletal muscle fibers. J. Appl. Physiol. 2012, 112, 728–736. [Google Scholar] [CrossRef]
- Patterson, M.F.; Stephenson, G.M.; Stephenson, D.G. Denervation produces different single fiber phenotypes in fast- and slow-twitch hindlimb muscles of the rat. Am. J. Physiol. Cell Physiol. 2006, 291, C518–C528. [Google Scholar] [CrossRef]
- Talmadge, R.J.; Roy, R.R. Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J. Appl. Physiol. 1993, 75, 2337–2340. [Google Scholar] [CrossRef]
- Mizunoya, W.; Wakamatsu, J.-I.; Tatsumi, R.; Ikeuchi, Y. Protocol for high-resolution separation of rodent myosin heavy chain isoforms in a mini-gel electrophoresis system. Anal. Biochem. 2008, 377, 111–113. [Google Scholar] [CrossRef]







| Muscle | Genotype | Pt (mN/mm2) | TPT (s) | ½ RT (s) | Dx/Dt (mN/s) |
|---|---|---|---|---|---|
| EDL | WT/WT | 83.23 ± 9.3 | 0.018 ± 0.0005 | 0.015 ± 0.0005 | 3911 ± 539 |
| WT/MUT | 63.69 ± 9.2 | 0.017 ± 0.0005 | 0.015 ± 0.0005 | 3944 ± 457 | |
| MUT/MUT | 61.3 ± 10.7 | 0.017 ± 0.0004 | 0.014 ± 0.0005 | 3952 ± 661 | |
| SOL | WT/WT | 10.4 ± 1.08 | 0.031 ± 0.003 | 0.074 ± 0.018 | 495.6 ± 52.2 |
| WT/MUT | 10.01 ± 0.39 | 0.033 ± 0.003 | 0.096 ± 0.017 | 442.4 ± 27.7 | |
| MUT/MUT | 8.96 ± 2.02 | 0.031 ± 0.001 | 0.071 ± 0.011 | 427.2 ± 92.1 |
| Muscle | Genotype | N | n | Fibre Types | Type I + Type II | ||
|---|---|---|---|---|---|---|---|
| Mice | Fibres | Type II | Type I | Mixed | (% Total Fibres) | ||
| EDL | WT/WT | 4 | 16 | 14 (87.5%) | 1 (6.3%) | 1 (6.3%) | 93.8% |
| WT/MUT | 5 | 14 | 12 (85.7%) | 2 (14.3%) | 0 (0%) | 100% | |
| MUT/MUT | 3 | 12 | 9 (75%) | 3 (25%) # | 0 (0%) | 100% | |
| SOL | WT/WT | 4 | 21 | 10 (47.6% | 9 (42.9%) | 2 (9.5%) | 90.5% |
| WT/MUT | 5 | 15 | 4 (26.7%) | 8 (53.3%) | 3 (20%) | 80% | |
| MUT/MUT | 3 | 15 | 7 (46.7%) | 4 (26.7%) | 4 (26.7%) # | 73.3% # | |
| Muscle | Genotype | N Mice | n Fibres | pCa50 | h |
|---|---|---|---|---|---|
| EDL | WT/WT | 4 | 13 | 5.794 ± 0.017 | 5.725 ± 0.536 |
| WT/MUT | 5 | 12 | 5.849 ± 0.016 | 5.704 ± 0.491 | |
| MUT/MUT | 3 | 9 | 5.862 ± 0.009 # | 5.872 ± 0.249 | |
| SOL | WT/WT | 4 | 18 | 5.789 ± 0.026 | 3.984 ± 0.224 |
| WT/MUT | 5 | 12 | 5.858 ± 0.019 | 3.660 ± 0.296 | |
| MUT/MUT | 3 | 12 | 5.868 ± 0.029 # | 4.025 ± 0.326 |
| Genotype | Young | Middle-Aged | Old | |
|---|---|---|---|---|
| rel Po | WT/WT | 1.520 ± 0.208 (n = 14) # | 1.343 ± 0.189 (n = 16) # | 1.169 ± 0.235 (n = 8) |
| WT/MUT | 1.990 ± 0.628 (n = 16) | 2.073 ± 0.721 (n = 12) | 2.049 ± 0.601 (n = 6) | |
| MUT/MUT | 2.066 ± 0.799 (n = 12) | 1.649 ± 0.463 (n = 14) | 2.792 ± 0.961 (n = 6) # Ω | |
| rel To | WT/WT | 1.111 ± 0.088 (n = 14) # | 1.068 ± 0.0719 (n = 16) | 1.049 ± 0.110 (n = 8) |
| WT/MUT | 1.251 ± 0.091 (n = 16) # | 1.142 ± 0.054 (n = 12) # | 1.250 ± 0.066 (n = 6) # | |
| MUT/MUT | 1.032 ± 0.146 (n = 12) | 1.032 ± 0.058 (n = 14) | 1.648 ± 0.569 (n = 6) | |
| rel Fo | WT/WT | 1.399 ± 0.187 (n = 14) # | 1.405 ± 0.193 (n = 16) # | 1.043 ± 0.158 (n = 8) |
| WT/MUT | 1.363 ± 0.312 (n = 16) | 1.697 ± 0.576 (n = 12) | 1.700 ± 0.619 (n = 6) | |
| MUT/MUT | 2.751 ±1.795 (n = 12) | 1.561 ± 0.428 (n = 14) | 1.628 ± 0.237 (n = 6) # Ω |
| Genotype | Young | Middle-Aged | Old | ||||
|---|---|---|---|---|---|---|---|
| 10 µM caff | 10 mM caff | 10 µM caff | 10 mM caff | 10 µM caff | 10 mM caff | ||
| rel Po | WT/WT | 1.061 ± 0.211 | 13.010 ± 3.716 # | 1.480 ± 0.143 # | 12.652 ± 2.745 # | 1.215 ± 0.140 | 6.567 ± 1.541 # |
| WT/MUT | 1.630 ± 0.299 # | 15.425 ± 3.854 # | 1.610 ± 0.366 # | 9.766 ± 3.086 # | 1.998 ± 0.881 | 3.848 ± 0.386 # | |
| MUT/MUT | 1.164 ± 0.315 | 13.882 ± 3.643 # | 1.322 ± 0.194 | 12.615 ± 3.458 # | 5.234 ± 1.741 # Ω | 19.066 ± 5.560 # Ω | |
| rel Tc | WT/WT | 1.425 ± 0.228 | 0.209 ± 0.056 # | 0.964 ± 0.216 | 0.199 ± 0.038 # | 0.916 ± 0.081 | 0.504 ± 0.212 # |
| WT/MUT | 1.035 ± 0.197 | 0.309 ± 0.087 # | 0.786 ± 0.071 # | 0.356 ± 0.099 # | 1.083 ± 0.327 | 0.316 ± 0.047 # | |
| MUT/MUT | 1.444 ± 0.278 | 0.166 ± 0.033 # | 0.942 ± 0.099 | 0.235 ± 0.035 # | 0.746 ±0.2696 | 0.371 ± 0.241 # | |
| rel Fo | WT/WT | 0.995 ± 0.178 | 9.026 ± 2.554 # | 1.374 ± 0.134 # | 7.061 ± 1.251 # | 1.170 ± 0.134 | 6.702 ± 3.105 # |
| WT/MUT | 1.481 ± 0.260 # | 6.703 ± 1.609 # | 1.706 ± 0.398 # | 7.119 ± 2.140 # | 1.910 ± 0.787 | 3.657 ± 0.938 # | |
| MUT/MUT | 0.885 ± 0.286 | 9.639 ± 4.412 # | 1.232 ± 0.136 | 6.347 ± 0.327 # | 3.857 ± 1.625 | 8.155 ± 1.656 # | |
| EDL Group A µm2 | EDL Group B µm2 | SOL Group A µm2 | SOL Group B µm2 | |
|---|---|---|---|---|
| WT/WT | 808.9 ± 54.4 | 270.5 ± 28.9 @ | 645 ± 24 # | 322 |
| (n = 14) | (n = 14) | (n = 14) | (n = 1 of 14) | |
| WT/MUT | 713.8 ± 56.2 * | 270.5 ± 28.8 @ | 635 ± 32 # | 354 ± 67 |
| (n = 15) | (n = 15) | (n = 15) | (n = 3 of 15) | |
| MUT/MUT | 670.0 ± 31.4 * | 238.5 ± 10.42 @ | 566 ± 24 *# | 197 |
| (n = 10) | (n = 10) | (n = 10) | (n = 1 of 10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thekkedam, C.G.; Dutka, T.L.; Van der Poel, C.; Burgio, G.; Dulhunty, A.F. The RyR1 P3528S Substitution Alters Mouse Skeletal Muscle Contractile Properties and RyR1 Ion Channel Gating. Int. J. Mol. Sci. 2024, 25, 434. https://doi.org/10.3390/ijms25010434
Thekkedam CG, Dutka TL, Van der Poel C, Burgio G, Dulhunty AF. The RyR1 P3528S Substitution Alters Mouse Skeletal Muscle Contractile Properties and RyR1 Ion Channel Gating. International Journal of Molecular Sciences. 2024; 25(1):434. https://doi.org/10.3390/ijms25010434
Chicago/Turabian StyleThekkedam, Chris G., Travis L. Dutka, Chris Van der Poel, Gaetan Burgio, and Angela F. Dulhunty. 2024. "The RyR1 P3528S Substitution Alters Mouse Skeletal Muscle Contractile Properties and RyR1 Ion Channel Gating" International Journal of Molecular Sciences 25, no. 1: 434. https://doi.org/10.3390/ijms25010434