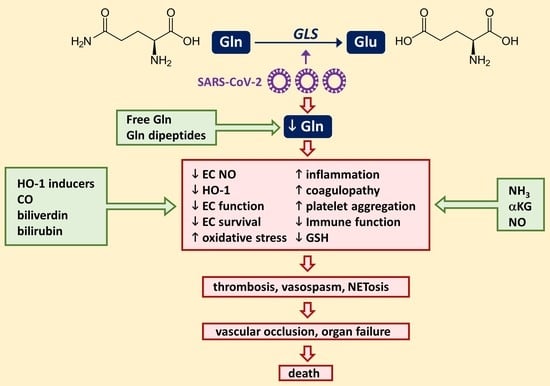
Glutamine Deficiency Promotes Immune and Endothelial Cell Dysfunction in COVID-19
Abstract
:1. Introduction
2. Glutamine Metabolism
3. Role of Glutamine in Immune Cells
4. Role of Glutamine in Vascular Health
5. Vascular Disease in COVID-19
6. Glutamine Deficiency in COVID-19
7. Strategies Targeting Glutamine in COVID-19
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus Disease (COVID) Dashboard. 2023. Available online: http:/covid19.who.int (accessed on 28 March 2023).
- Mukra, R.; Krishan, K.; Kanchan, T. Possible modes of transmission of novel coronavirus SARS-COVID-2: A review. Acta Bio. Med. 2020, 91, e2020036. [Google Scholar]
- Beyerstedt, S.; Casaro, E.B.; Rangel, E.B. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Z.; Poh, C.M.; Renia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunology, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- McFadyen, J.D.; Stevens, H.; Peter, K. The emerging threat of (micro) thrombosis in COVID-19 and its therapeutic implications. Circ. Res. 2020, 127, 571–587. [Google Scholar] [CrossRef]
- Nazerian, Y.; Ghasemi, M.; Yassaghi, Y.; Nazerian, A.; Hashemi, S.M. Role of SARS-CoV-2-induced cytokine storm in multi-organ failure: Molecular pathways and potential therapeutic options. Int. Immunopharmacol. 2022, 113, 109428. [Google Scholar] [CrossRef]
- Rozga, M.; Cheng, F.W.; Moloney, L.; Handu, D. Effects of micronutrients and conditional amino acids on COVID-19-related outcomes: An evidence analysis center scoping review. J. Acad. Nutr. Diet. 2021, 121, 1354–1363. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y. Potential interventions for novel coronavirus in China: A systematic review. J. Med. Virol. 2020, 92, 479–490. [Google Scholar] [CrossRef]
- Cheng, R.Z. Can early and high vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Med. Drug Discov. 2020, 5, 100028. [Google Scholar] [CrossRef]
- Laviano, A.; Koverech, A.; Zanetti, M. Nutritional support in the time of SARS-CoV-2 (COVID-19). Nutrition 2020, 74, 110834. [Google Scholar] [CrossRef]
- Roth, R. Nonnutritive effects of glutamine. J. Nutr. 2008, 138, 2025S–2031S. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.-L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef]
- Cruzat, V.; Rogero, M.M.; Keane, K.N.; Curi, R.; Newsholme, P. Glutamine: Metabolism and immune function, supplementation, and clinical translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- Li, P.; Wu, G. Important roles of amino acids in immune responses. Br. J. Nutr. 2022, 127, 398–402. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, Z.; Li, P.; Zhang, Z.; Zeng, M.; Liang, Z.; Li, D.; Wang, L.; Chen, Y.; Liang, Y.; et al. Reprogramming of glutamine metabolism and its impact on immune response in the tumor microenvironment. Cell Commun. Signal. 2022, 20, 114. [Google Scholar] [CrossRef]
- Van der Hulst, R.R.; van Kreel, B.K.; von Meyenfeldt, M.F.; Brummer, R.J.; Arends, J.W.; Deutz, N.E.; Soeters, P.B. Glutamine and the preservation of gut integrity. Lancet 1993, 341, 1363–1365. [Google Scholar] [CrossRef]
- Melis, G.C.; ter Wengel, N.; Boelens, P.G.; van Leeuwen, P.A.M. Glutamine: Recent developments in research on the clinical significance of glutamine. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 59–70. [Google Scholar] [CrossRef]
- Morlion, B.J.; Stehle, P.; Wachtler, P.; Siedhoff, H.; Koller, M.; Konig, W.; Furst, P.; Puchstein, C. Total parenteral nutrition with glutamine dipeptide after major abdominal surgery: A randomized, double-blind, controlled study. Ann. Surg. 1998, 227, 302–308. [Google Scholar] [CrossRef]
- McBurney, M.; Young, L.S.; Ziegler, T.R.; Wimore, D.W. A cost-evaluation of glutamine-supplemented parenteral nutrition in adult bone marrow transplant patients. J. Am. Diet. Assoc. 1994, 94, 1263–1266. [Google Scholar] [CrossRef]
- Ziegler, T.R.; Bye, R.L.; Persinger, R.L.; Young, L.; Antin, J.; Wilmore, D.W. Effects of glutamine supplementation on circulating lymphocytes after bone marrow transplantation: A pilot study. Am. J. Med. Sci. 1998, 315, 4–10. [Google Scholar] [CrossRef]
- Durante, W. The emerging role of L-glutamine in cardiovascular health and disease. Nutrients 2019, 11, 2092. [Google Scholar] [CrossRef]
- Durante, W. Amino acids in circulatory function and health. Adv. Exp. Med. Biol. 2020, 1265, 39–56. [Google Scholar] [PubMed]
- Shen, B.; Yi, X.; Sun, Y.; Bi, X.; Du, J.; Zhang, C.; Quan, S.; Zhang, F.; Sun, R.; Qian, L.; et al. Proteomic and metabolomic characterization of COVID-19 patient sera. Cell 2020, 182, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Buhtia, Y.D.; Ganapathy, V. Glutamine transorters in mammalian cells and their functions in physiology and cancer. Biochem. Biophys. Acta 2016, 1863, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Pochini, L.; Scalise, M.; Galluccio, M.; Indiveri, C. Membrane transporters for the special amino acid glutamine: Structure/function relationships and relevance to human health. Front. Chem. 2015, 2, 61. [Google Scholar] [CrossRef]
- Kandasamy, P.; Gyimesi, G.; Kanai, Y.; Hediger, M.A. Amino acid transporters revisited: New views in health and disease. Trends Biochem. Sci. 2018, 43, 752–789. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Liu, X.; Cheng, C.; Yu, W.; Yi, P. Metabolism of amino acids in cancer. Front. Cell Dev. Biol. 2021, 8, 603837. [Google Scholar] [CrossRef]
- Bertero, T.; Oldham, W.M.; Cottrill, K.A.; Pisano, S.; Vanderpool, R.R.; Yu, Q.; Zhao, J.; Tai, Y.; Tang, Y.; Zhang, Y.Y.; et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J. Clin. Investig. 2016, 126, 3313–3335. [Google Scholar] [CrossRef]
- Peyton, K.J.; Liu, X.M.; Yu, Y.; Yates, B.; Behnammanesh, G.; Durante, W. Glutaminase-1 stimulates the proliferation, migration, and survival of human endothelial cells. Biochem. Pharmacol. 2018, 156, 204–214. [Google Scholar] [CrossRef]
- Carr, E.L.; Kelman, A.; Wu, G.S.; Gopaul, R.; Senkevitch, E.; Aghvanyan, A.; Turay, A.M.; Frauwirth, K.A. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J. Immunol. 2010, 185, 1037–1044. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, C.; Wu, Y.Q.; Zhuang, G.; Ye, Z.; Zhang, C.S.; Lin, S.-C. Glutaminase GLS1 senses glutamine availability in a non-enzymatic manner triggering mitochondrial fusion. Cell Res. 2018, 28, 865–867. [Google Scholar] [CrossRef]
- Liu, X.M.; Peyton, K.J.; Durante, W. Ammonia promotes endothelial cell survival via the heme oxygenase-1 mediated release of carbon monoxide. Free Radic. Biol. Med. 2017, 102, 37–46. [Google Scholar] [CrossRef]
- Eng, C.H.; Yu, K.; Lucas, J.; White, E.; Abraham, R.T. Ammonia derived from glutaminolysis is a diffusible regulator of autophagy. Sci. Signal. 2010, 3, ra31. [Google Scholar] [CrossRef]
- Abusneina, A.; Gauthier, E.R. Ammonium ions improve the survival of glutamine-starved hybridoma cells. Cell Biosci. 2016, 6, 23. [Google Scholar] [CrossRef]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef]
- Spittler, A.; Holzer, S.; Oehler, R.; Boltz-Nitulescu, G.; Roth, E. A glutamine deficiency impairs the function of cultured human monocytes. Clin. Nutr. 1997, 16, 97–99. [Google Scholar] [CrossRef]
- Yaqoob, P.; Calder, P.C. Cytokine production by human peripheral blood mononuclear cells: Differential sensitivity to glutamine availability. Cytokine 1998, 10, 790–794. [Google Scholar] [CrossRef]
- Field, C.J.; Johnson, I.R.; Schley, P.D. Nutrients and their role in host resistance to infection. J. Leukoc. Biol. 2002, 71, 16–32. [Google Scholar] [CrossRef]
- Murphy, C.; Newsholme, P. Importance of glutamine metabolism in murine macrophages and human monocytes to L-arginine biosynthesis and rates of nitrite or urea production. Clin. Sci. 1998, 89, 397–407. [Google Scholar] [CrossRef]
- Lisi, F.; Zelikin, A.N.; Chandrawati, R. Nitric oxide to fight viral infections. Adv. Sci. 2021, 8, 2003895. [Google Scholar] [CrossRef]
- Akaberi, D.; Krambrich, J.; Ling, J.; Luni, C.; Hedenstierna, G.; Jarhult, J.D.; Lennerstrand, J.; Lundkvist, A. Mitigation of the replication of SARS-CoV-2 by nitric oxide in vitro. Redox Biol. 2020, 37, 101734. [Google Scholar] [CrossRef]
- Marti I Lindez, A.-A.; Reith, W. Arginine-dependent immune responses. Cell. Mol. Life Sci. 2021, 78, 5303–5324. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Rice, C.M.; Palmieri, E.M.; Taylor, P.R.; Kuhns, D.B.; McVicar, D.W. Peritoneal tissue-resident macrophages are metabolically poised to engage microbes using tissue-niche fuels. Nat. Commun. 2017, 8, 2074. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.S.; Wang, H.; Li, X.; Chao, T.; Teav, T.; Christen, S.; Di Conza, G.; Cheng, W.C.; Chau, C.H.; Vavakova, M.; et al. Alpha-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 2017, 18, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Franek, F.; Sramkova, K. Protection of B lymphocyte hybridoma against starvation-induced apoptosis: Survival signal role of some amino acids. Immunol. Lett. 1996, 52, 139–144. [Google Scholar] [CrossRef]
- Iperi, C.; Bordron, A.; Dueymes, M.; Pers, J.-O.; Jamin, C. Metabolic program of regulatory B lymphocytes and influence in the control of malignant and autoimmune situations. Front. Immunol. 2021, 12, 735463. [Google Scholar] [CrossRef]
- Kedia-Mehta, N.; Finlay, D.K. Competition for nutrients and its role in controlling immune responses. Nat. Commun. 2019, 10, 2123. [Google Scholar] [CrossRef]
- Colombo, S.L.; Palacios-Callender, M.; Frakich, N.; De Leon, J.; Schmitt, C.A.; Boorn, L.; Davis, N.; Moncada, S. Anaphase-promoting complex/cyclsome-Cdh1 coordinates glycolysis and glutaminolysis with transition to S-phase in human T-lymphocytes. Proc. Natl. Acad. Sci. USA 2010, 107, 18868–18873. [Google Scholar] [CrossRef]
- Sener, Z.; Cederkvist, F.H.; Volchenkov, R.; Holen, H.L.; Skalhegg, B.S. T helper cell activation and expansion in sensitive to glutamine inhibition under both hypoxic and normoxic conditions. PLoS ONE 2016, 11, e0160291. [Google Scholar] [CrossRef]
- Nakaya, M.; Xio, Y.; Zhou, X.; Chang, J.H.; Chang, M.; Cheng, X.; Blonska, M.; Lin, X.; Sun, S.-C. Inflammatory T cell responses rely on amino acid transporter Asct2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity 2014, 40, 692–705. [Google Scholar] [CrossRef]
- Klyz, D.; Tai, X.; Robert, P.A.; Craveiro, M.; Cretenet, G.; Oburoglu, L.; Mongellaz, C.; Floess, S.; Fritz, V.; Matias, M.I.; et al. Glutamine-dependent α-ketoglutarate production regulates the balance between T helper cell 1 and regulatory T cell generation. Sci. Signal. 2015, 8, ra97. [Google Scholar] [CrossRef]
- Xu, T.; Stewart, K.M.; Wang, X.; Liu, K.; Xie, M.; Ryu, J.K.; Li, K.; Ma, T.; Wang, H.; Ni, L.; et al. Metabolic control of TH17 and induced Treg cell balance by an epigenetic mechanism. Nature 2017, 548, 228–233. [Google Scholar] [CrossRef]
- Johnson, M.O.; Wolf, M.M.; Madden, M.Z.; Andrejeva, G.; Sugiura, A.; Contreras, D.C.; Maseda, D.; Liberti, M.V.; Paz, K.; Kishton, R.J.; et al. Distinct regulation of Th17 and Th1 cell differentiation by glutaminase-dependent metabolism. Cell 2018, 175, 1780–1795. [Google Scholar] [CrossRef]
- Best, S.A.; Gubser, P.M.; Sethumadhavan, S.; Kersbergen, A.; Abril, Y.L.N.; Goldfarb, J.; Sellers, K.; Abeysekera, W.; Garnham, A.L.; McDonald, J.A.; et al. Glutaminase inhibition impairs CD8 T cell activation in STK11-/Lkb1-deficient lung cancer. Cell Metab. 2022, 34, 874–887. [Google Scholar] [CrossRef]
- Agrati, C.; Sacchi, A.; Bordoni, V.; Cimini, E.; Notari, S.; Grassi, G.; Casetti, R.; Tartaglia, E.; Lalle, E.; D’Abramo, A.; et al. Expansion of myeloid-derived suppressor cells in patients with severe coronavirus disease (COVID-19). Cell Death Differ. 2021, 27, 3196–3207. [Google Scholar] [CrossRef]
- Oh, M.-H.; Sun, I.-H.; Zhao, L.; Leone, R.D.; Sun, I.-M.; Xu, W.; Collins, S.L.; Tam, A.J.; Blosser, R.L.; Patel, C.H.; et al. Targeting glutamine metabolism enhances tumor-specific immunity by modulating suppressive myeloid cells. J. Clin. Investig. 2020, 130, 3865–3884. [Google Scholar] [CrossRef]
- Hammami, I.; Chen, J.; Bronte, V.; DeCrescenzo, G.; Jolicoeur, M. L-glutamine is a key parameter in the immunosuppression phenomenon. Biochem. Biophys. Res. Commun. 2012, 425, 724–729. [Google Scholar] [CrossRef]
- Sun, H.-W.; Wu, W.-C.; Chen, H.-T.; Xu, Y.-T.; Yang, Y.-Y.; Chen, J.; Yu, X.-J.; Wang, Z.; Shuang, Z.-Y.; Zheng, L. Glutamine deprivation promotes the generation and mobilization of MDSCs by enhancing expression of G-CSF and GM-CSF. Front. Immunol. 2020, 11, 616367. [Google Scholar] [CrossRef]
- Shu, X.-L.; Yu, T.-T.; Kang, K.; Zhao, J. Effects of glutamine on markers of intestinal inflammatory response and mucosal permeability in abdominal surgery patients: A meta-analysis. Exp. Ther. Med. 2016, 12, 3499–3506. [Google Scholar] [CrossRef]
- Jimenez, R.V.; Kuznetsova, V.; Connelly, A.N.; Hel, Z.; Szalai, A.J. C-reactive protein promotes the expansion of myeloid-derived cells with suppressor functions. Front. Immunol. 2019, 10, 2183. [Google Scholar] [CrossRef]
- Pithon-Curi, T.C.; Schumacher, I.; Freitas, J.J.S.; Lagranha, C.; Newsholme, P.; Palanch, A.C.; Doi, S.Q.; Curi, R. Glutamine delays spontaneous apoptosis in neutrophils. Am. J. Physiol. Cell Physiol. 2003, 284, C1355–C1361. [Google Scholar] [CrossRef]
- Santos, A.C.A.; Hebeba, C.B.; Hastreiter, A.A.; de Oliveira, D.C.; Makiyama, E.N.; Farsky, S.H.P.; Borelli, P.; Fock, R.A. Exogenous glutamine impairs neutrophil migration into infections sites elicited by lipopolysaccharide by a multistep mechanism. Amino Acids 2019, 51, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kim, M.J.; Kim, Y.; Kim, H. Glutamine depreviation causes hydrogen peroxide induced-induced interleukin-8 expression via Jak1/Stat3 activation in gastric epithelial AGS cells. J. Cancer Prev. 2015, 20, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Leppkes, M.; Knopf, J.; Naschberger, E.; Lindemann, A.; Singh, J.; Herrmann, J.; Stürzl, M.; Staats, L.; Mahajan, A.; Schauer, C.; et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine 2020, 58, 102925. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Yarosz, E.L.; Andren, A.; Zhang, L.; Lyssiotis, C.A.; Chang, C.-H. NKT cells adopt a glutamine-addicted phenotype to regulate their homeostasis and function. Cell Rep. 2022, 41, 111516. [Google Scholar] [CrossRef]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef]
- Würtz, P.; Mäkinen, V.P.; Soininen, P.; Kangas, A.J.; Tukiainen, T.; Kettunen, J.; Savolainen, M.J.; Tammelin, T.; Viikari, J.S.; Rönnemaa, T.; et al. Metabolic signatures of insulin resistance in 7,098 young adults. Diabetes 2012, 61, 1372–1380. [Google Scholar] [CrossRef]
- Cheng, S.; Rhee, E.P.; Larson, M.G.; Lewis, G.D.; McCabe, E.L.; Shen, D.; Palma, M.J.; Roberts, L.D.; Dejam, A.; Souza, A.L.; et al. Metabolite profiling identifies pathways associated with cardiovascular risk in humans. Circulation 2012, 125, 2222–2231. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, F.B.; Ruiz-Canela, M.; Clish, C.B.; Dennis, C.; Salas-Salvado, J.; Hruby, A.; Liang, L.; Toledo, E.; Corella, D.; et al. Metabolites of glutamate metabolism are associated with incident cardiovascular events in the PREDIMED PREvencion con DIeta MEDiterranea (PREDIMED) trial. J. Am. Heart Assoc. 2016, 5, e003755. [Google Scholar] [CrossRef]
- Qi, L.; Qi, Q.; Prudente, S.; Mendonca, C.; Andreozzi, F.; Di Pietro, N.; Sturma, M.; Novelli, V.; Mannino, G.C.; Formoso, G.; et al. Association between a genetic variant of related to glutamic acid metabolism and coronary heart disease in type 2 diabetes. JAMA 2013, 310, 8212–8828. [Google Scholar] [CrossRef]
- Ma, W.; Heianza, Y.; Huang, T.; Wang, T.; Sun, D.; Zheng, Y.; Hu, F.B.; Rexrode, K.M.; Manson, J.; Qi, L. Dietary glutamine, glutamate, and mortality: Two large prospective studies in US men and women. Int. J. Epidemiol. 2018, 47, 311–320. [Google Scholar] [CrossRef]
- Safi, S.Z.; Batumalaie, K.; Mansor, M.; Chinna, K.; Mohan, S.; Karimian, H.; Qvist, R.; Ashraf, M.A.; Yan, G.O. Glutamine treatment attenuates hyperglycemia-induced mitochondrial stress and apoptosis in umbilical vein endothelial cells. Clinics 2015, 70, 569–576. [Google Scholar] [CrossRef]
- Hinshaw, D.B.; Burger, J.M. Protective effect of glutamine on endothelial cell ATP in oxidant injury. J. Surg. Res. 1990, 49, 222–227. [Google Scholar] [CrossRef]
- Parolari, A.; Sala, R.; Antona, C.; Bussolati, O.; Alamanni, F.; Mezzadri, P.; Dall’Asta, V.; Gazzola, G.C.; Biglioli, P. Hypertonicity induces injury to cultured human endothelium: Attenuation by glutamine. Ann. Thorac. Surg. 1997, 64, 1770–1775. [Google Scholar] [CrossRef]
- Sanchez, E.L.; Carroll, P.A.; Thalhofer, A.B.; Lagunoff, M. Latent KSHV infected endothelial cells are glutamine addicted and require glutaminolysis for survival. PLoS Pathog. 2015, 11, e1005052. [Google Scholar] [CrossRef]
- Hsu, C.S.; Chou, S.Y.; Liang, S.J.; Changt, C.Y.; Yeh, S.L. Effect of physiologic levels of glutamine on ICAM-1 expression in endothelial cells activated by preeclamptic plasma. J. Reprod. Med. 2006, 51, 193–198. [Google Scholar]
- Hou, Y.C.; Hsu, C.S.; Yeh, C.L.; Chiu, W.C.; Pai, M.H.; Yeh, S.L. Effect of glutamine on adhesion molecule expression and leukocyte transmigration in endothelial cells exposed to arsenic. J. Nutr. Biochem. 2005, 16, 700–704. [Google Scholar] [CrossRef]
- Yeh, C.L.; Hsu, C.S.; Chen, S.C.; Pai, M.H.; Yeh, S.L. Effect of glutamine on cellular adhesion molecule expression and leukocyte transmigration in endothelial cells stimulated by plasma or peritoneal drain fluid from a surgical patient. Shock 2006, 25, 236–240. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.; Ogando, D.G.; Li, S.; Feng, M.; Price, F.W., Jr.; Tennessen, J.M.; Bonanno, J.A. Glutaminolysis is essential for energy production and ion transport in human corneal endothelium. EBiomedicine 2017, 16, 292–301. [Google Scholar] [CrossRef]
- Unterluggauer, H.; Mazurek, B.; Lener, E.; Hutter, E.; Eigenbrodt, W.; Zwersche, W.; Jansen-Durr, P. Premature senescence of human endothelial cells induced by inhibition of glutaminase. Biogerentology 2008, 9, 247–259. [Google Scholar] [CrossRef]
- Su, S.T.; Yeh, C.L.; Hou, Y.C.; Pai, M.H.; Yeh, S.L. Dietary glutamine supplementation enhances endothelial progenitor cell mobilization in streptozotocin-induced diabetic mice subjected to limb ischemia. J. Nutr. Biochem. 2017, 40, 86–94. [Google Scholar] [CrossRef]
- Pai, M.H.; Shih, Y.M.; Shih, J.M.; Yeh, C.L. Glutamine administration modulates endothelial progenitor cell and lung injury in septic mice. Shock 2016, 46, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Addabbo, F.; Chen, Q.; Patel, D.P.; Rabadi, M.; Ratliff, B.; Zhang, F.; Jasmin, J.F.; Wolin, M.; Lisanti, M.; Goligorsky, M.S. Glutamine supplementation alleviates vasculopathy and corrects metabolic profile in an in vivo model of endothelial cell dysfunction. PLoS ONE 2013, 8, e65458. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.C.; Patterson, M.; Dudenbostel, T.; Ccalhoun, D.; Gower, B. Effects of 6 month supplementation with β-hydroxy-β-methybutyrate, glutamine and arginine on vascular endothelial function of older adults. Eur. J. Clin. Nutr. 2016, 70, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Schachter, D. L-Glutamine in vitro regulates rat aortic glutamate content and modulates nitric oxide formation and contractility response. Am. J. Physiol. Cell Physiol. 2007, 293, C142–C151. [Google Scholar] [CrossRef] [PubMed]
- Rohlenova, K.; Veys, K.; Miranda-Santos, I.; De Bock, K.; Carmeliet, P. Endothelial cell metabolism in health and disease. Trends Cell Biol. 2018, 28, 224–236. [Google Scholar] [CrossRef]
- Kim, B.; Li, J.; Jang, C.; Arany, Z. Glutamine fuels proliferation but not migration of endothelial cells. EMBO J. 2017, 36, 2321–2333. [Google Scholar] [CrossRef]
- Huang, H.; Vandekeere, S.; Kalucka, J.; Bierhansl, L.; Zecchin, A.; Bruning, U.; Visnagri, A.; Yuldasheva, N.; Goveia, J.; Cruys, B.; et al. Role of glutamine and interlinked asparagine metabolism in vessel formation. EMBO J. 2017, 36, 2334–2352. [Google Scholar] [CrossRef]
- Koyama, S.; Yamashita, A.; Matsuura, Y.; Saito, Y.; Maekawa, K.; Gi, T.; Kitamura, K.; Asada, Y. Intracellular glutamine level determines vascular smooth muscle cell-derived thrombogenicity. Atherosclerosis 2021, 328, 62–73. [Google Scholar] [CrossRef]
- Mushtaq, M.; Kim, U.-H. The role of glutamine in the prevention of ultraviolet-C-induced platelet activation. Biochem. Res. Int. 2020, 2020, 8853696. [Google Scholar] [CrossRef]
- Morrell, C.N.; Sun, H.; Ikeda, M.; Beique, J.-C.; Swaim, A.M.; Mason, E.; Martin, T.V.; Thompson, L.E.; Gozen, O.; Ampagoomian, D.; et al. Glutamate mediates platelet activation through the AMPA receptor. J. Exp. Med. 2008, 205, 574–584. [Google Scholar] [CrossRef]
- Gautam, D.; Tiwari, A.; Chaurasia, R.N.; Dash, D. Glutamate induces synthesis of thrombogenic peptides and extracellular vesicle release from human platelets. Sci. Rep. 2019, 9, 8346. [Google Scholar] [CrossRef]
- Ayer, A.; Zarjou, A.; Agarwal, A.; Stocker, R. Heme oxygenases in cardiovascular health and disease. Physiol. Rev. 2016, 96, 1449–1508. [Google Scholar] [CrossRef]
- Durante, W.; Johnson, F.K.; Johnson, R.A. Role of carbon monoxide in cardiovascular function. J. Cell. Mol. Med. 2006, 10, 672–686. [Google Scholar] [CrossRef]
- Durante, W. Targeting heme oxygenase-1 in vascular disease. Curr. Drug. Ther. 2010, 11, 1504–1516. [Google Scholar] [CrossRef]
- Durante, W. Targeting heme oxygenase-1 in the arterial response to injury and disease. Antioxidants 2020, 9, 829. [Google Scholar] [CrossRef]
- Korthuis, R.J.; Durante, W. Heme oxygenase-1: A pluripotent sentinel limiting systemic inflammatory response to extremity ischemia and reperfusion. Crit. Care Med. 2005, 33, 2321–2333. [Google Scholar] [CrossRef]
- Gu, S.X.; Tyagi, T.; Jain, K.; Gu, V.W.; Lee, S.H.; Hwa, J.M.; Kwan, J.M.; Krause, D.S.; Lee, A.I.; Halene, S.; et al. Thrombocytopathy and endothelialiopathy: Crucial contributors to COVID-19 thromboinflammation. Nat. Rev. Cardiol. 2021, 18, 194–209. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, Q.; Wang, Y.; Wu, Y.; Xu, J.; Yu, Y.; Shange, Y. Thrombocytopenia and its association with mortality in patients with COVID-19. J. Thromb. Haemostas. 2020, 18, 1469–1472. [Google Scholar] [CrossRef]
- Lippi, G.; Favoloro, E.J. D-dimer is associated with severity of coronavirus disease 2019: A pooled analysis. J. Thromb. Haemostas. 2020, 18, 876–878. [Google Scholar] [CrossRef]
- Lippi, G.; Phlebani, M.; Henry, B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin. Chem. Acta 2020, 506, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Chen, S.; Li, X.; Liu, S.; Wang, F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb. Res. 2020, 191, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, D.; Sperhake, J.P.; Lutgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schröder, A.S.; et al. Autopsy findings and venous thromboembolism in patients with COVID-19: A prospective cohort study. Ann. Intern. Med. 2020, 173, 268. [Google Scholar] [CrossRef] [PubMed]
- Carsana, L. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: A two-center descriptive study. Lancet Infect. Dis. 2020, 20, 1135–1140. [Google Scholar] [CrossRef]
- Menter, T.; Haslbauer, J.D.; Nienhold, R.; Savic, S.; Hopfer, H.; Deigendesch, N.; Frank, S.; Turek, D.; Willi, N.; Pargger, H.; et al. Post-mortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology 2020, 77, 198–209. [Google Scholar] [CrossRef]
- Fox, S.E.; Akmatbekov, A.; Harbert, J.L.; Li, G.; Brown, J.Q.; Heide, R.S.V. Pulmonary and cardiac pathology in African American patients with COVID-19: An autopsy series from New Orleans. Lancet Respir. Med. 2020, 8, 681–688. [Google Scholar] [CrossRef]
- Modin, D.; Claggett, B.; Sindet-Pedersen, C.; Lassen, M.C.H.; Skaarup, K.G.; Jensen, J.U.S.; Fralick, M.; Schou, M.; Lamberts, M.; Gerds, T.; et al. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation 2020, 142, 2080–2082. [Google Scholar] [CrossRef]
- Oxley, T.J.; Mocco, J.; Majidi, S.; Kellner, C.P.; Shoirah, H.; Singh, I.P.; De Leacy, R.A.; Shigematsu, T.; Ladner, T.R.; Yaeger, K.A.; et al. Large-vessel stroke as a presenting feature of COVID-19 in the young. N. Engl. J. Med. 2020, 382, e60. [Google Scholar] [CrossRef]
- Gonzalez-Urquijo, M.; Gonzalez-Rayes, J.M.; Castro-Varela, A.; Hinojosa-Gonzalez, D.E.; Ramos-Cazares, R.E.; Vazquez-Garza, E.; Paredes-Vazquez, J.G.; Castillo-Perez, M.; Jerjes-Sanchez, C.; Fabiani, M.A.; et al. Unexpected arterial thrombosis and acute limb ischemia in COVID-19 patients. Results from the Ibero-Latin American acute arterial thrombosis registry in COVID-19: (ARTICO-19). Vascular 2021, 30, 1107–1114. [Google Scholar] [CrossRef]
- Rapkiewicz, A.V.; Mai, X.; Carsons, S.E.; Pittaluga, S.; Kleiner, D.E.; Berger, J.S.; Thomas, S.; Adler, N.; Charytan, D.; Gasmi, B.; et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinical 2020, 24, 100434. [Google Scholar] [CrossRef]
- Viola, F.; Pignatelli, P.; Cammisotto, V.; Bartimoccia, S.; Carnevale, R.; Nocella, C. COVID-19 and thrombosis: Clinical features, mechanism of disease, and therapeutic implications. Kardiol. Pol. 2021, 79, 1197–1205. [Google Scholar] [CrossRef]
- Fanning, J.P.; Weaver, N.; Fanning, R.B.; Griffee, M.J.; Cho, S.-M.; Panigada, M.; Obonyo, N.G.; Zaaqoq, A.M.; Rando, H.; Chia, Y.W.; et al. COVID-19 Critical Care Consortium. Hemorrhagic, disseminated intravascular coagulopathy, and thrombosis complications among critically ill patients with COVID-19: An International COVID-19 Critical Care Consortium Study. Crit. Care Med. 2023, 51, e005798. [Google Scholar] [CrossRef]
- Flaumenhaft, R.; Enjyoi, K.; Schmaier, A.A. Vasculopathy in COVID-19. Blood 2022, 140, 222–235. [Google Scholar] [CrossRef]
- Nicosia, R.F.; Ligresti, G.; Caporarello, N.; Akilesh, S.; Ribatti, D. COVID-19 Vasculopathy: Mounting evidence for an indirect mechanism of endothelial injury. Am. J. Pathol. 2021, 191, 1374–1384. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, K.Y.; Huang, Y.; Liu, K.O. Endothelial contribution to COVID-19: An update on mechanisms and therapeutic implications. J. Mol. Cell. Cardiol. 2022, 164, 69–82. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Havervich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis, in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Escher, R.; Breakey, N.; Lammle, B. Severe COVID-19 infection associated with endothelial activation. Thromb. Res. 2020, 190, 62. [Google Scholar] [CrossRef]
- Fraser, D.D.; Patterson, E.K.; Daley, M.; Cepinskas, G.; on behalf of the Lawson COVID-19 Study Team. Case report: Inflammation and endothelial injury profiling of COVID-19 pediatric multisystem inflammatory syndrome (MIS-C). Front. Pediatr. 2021, 9, 597926. [Google Scholar] [CrossRef]
- Crippa, S.; Kagi, G.; Graf, L.; Meyer Sauteur, P.M.; Kohler, P. Stroke in young adult with mild COVID-19 suggesting endothelialitis. New Microbes New Infect. 2020, 38, 100781. [Google Scholar] [CrossRef]
- Neri, T.; Nieri, D.; Celi, A. P-selectin blockade in COVID-19-related ARDS. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L1237–L1238. [Google Scholar] [CrossRef] [PubMed]
- Goshua, G.; Pine, A.B.; Meizlish, M.L.; Chang, C.H.; Zhang, H.; Bahel, P.; Baluha, A.; Bar, N.; Bona, R.D.; Burns, A.J.; et al. Endotheliopathy in COVID-19-associated coagulopathy: Evidence from a single centre, cross-sectional study. Lancet Haematol. 2020, 7, e575–e582. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, S.; Fogagnolo, A.; Campo, G.; Zucchetti, O.; Verri, M.; Ottaviani, I.; Tunstall, T.; Grasso, S.; Scaramuzzo, V.; Murgolo, F.; et al. Markers of endothelial and epithelial pulmonary injury in mechanically-ventilated COVID-19 ICU patients. Crit. Care 2021, 25, 74. [Google Scholar] [CrossRef] [PubMed]
- Al-Farabi, M.J.; Nugraha, R.A.; Marsudi, B.A.; Azmi, Y. Biomarkers of endothelial dysfunction and outcomes in caronavirus disease 2019 (COVID-19) patients: A systemic review and meta-analysis. Microvasc. Res. 2021, 138, 104224. [Google Scholar]
- Sabioni, L.R.; Tibirica, E.; Lamas, C.C.; Amorim, G.D.; De Lorenzo, A. Systemic microvascular dysfunction in COVID-19. Am. J. Cardiovasc. Dis. 2020, 10, 386–391. [Google Scholar]
- Sabioni, L.; De Lorenzo, A.; Lamas, C.; Mucillo, F.; Castro-Faria-Neto, H.C.; Estato, V.; Tibirica, E. Systemic microvascular endothelial dysfunction and disease severity in COVID-19 patients: Evaluation by laser Doppler perfusion monitoring and cytokine/chemokine analysis. Microvasc. Res. 2021, 134, 104119. [Google Scholar] [CrossRef]
- Ratchford, S.M.; Stickford, J.L.; Province, V.M.; Stute, N.; Augenreich, M.A.; Koontz, L.K.; Bobo, L.K.; Stickford, A.S.L. Vascular alterations among young adults with SARS-CoV-2. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H404–H410. [Google Scholar] [CrossRef]
- Sabioni, L.; De Lorenzo, A.; Castro-Faria-Neto, H.C.; Estato, V.; Tibirica, E. Long-term assessment of systemic microcirculatory function and plasma cytokines after coronavirus disease 2019 (COVID-19). Braz. J. Infect. Dis. 2023, 27, 102719. [Google Scholar] [CrossRef]
- Dominic, P.; Ahmad, J.; Bhandari, R.; Pardue, S.; Solorzano, J.; Jaisingh, K.; Watts, M.; Bailey, S.R.; Orr, A.W.; Kevil, C.G.; et al. Decreased availability of nitric oxide and hydrogen sulfide is a hallmark of COVID-19. Redox Biol. 2021, 43, 101982. [Google Scholar] [CrossRef]
- Yaghoubi, N.; Youssefi, M.; Jabbari Azad, F.; Farzad, F.; Yavari, Z.; Zahedi Avval, F. Total antioxidant capacity as a marker of severity of COVID-19 infection: Possible prognostic and therapeutic clinical application. J. Med. Virol. 2022, 94, 1558–1565. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, D.; Song, J.W.; Zullo, J.; Lipphardt, M.; Coneh-Gould, L.; Goligorsky, M.S. Endothelial cell dysfunction and the glycocalyx—A vicious circle. Matrix Biol. 2019, 71–72, 421–431. [Google Scholar] [CrossRef]
- Fraser, D.D.; Patterson, E.K.; Slessarev, M.; Gill, S.E.; Martinc, C.; Daley, M.; Miller, M.R.; Patel, M.A.; Santos, C.C.D.; Bosma, K.J.; et al. Endothelial injury and glycocalyx degradation in critically ill coronavirus disease 2019 patients: Implications for microvascular platelet aggregation. Crit. Care Explor. 2020, 2, e0194. [Google Scholar] [CrossRef]
- Du Preez, H.N.; Aldous, C.; Hayden, M.R.; Kruger, H.G.; Lin, J. Pathogenesis of COVID-19 described through the lens of undersulfated and degraded epithelial and endothelial glycocalyx. FASEB J. 2022, 36, e22052. [Google Scholar] [CrossRef]
- South, A.M.; Diz, D.I.; Chappell, M.C. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1084–H1090. [Google Scholar] [CrossRef]
- Sumerkan, M.C.; Kara, A.E.; Dogan, G.M.; Alyan, O. Spontaneous, severe, and diffuse coronary vasospasm in a patient with COVID-19. Anatol. J. Cardiol. 2021, 25, E36–E37. [Google Scholar] [CrossRef]
- Rivero, F.; Antuna, P.; Cuesta, J.; Alfonso, F. Severe coronary spasm in a COVID-19 patient. Catheter Cardiovasc. Interv. 2021, 97, E670–E672. [Google Scholar] [CrossRef]
- Wang, M.; Talon, A.; Saririan, M. COVID-19 cardiac arrest due to Prinzmetal’s angina in a previously normal heart. Clin. Case Rep. 2021, 9, e04205. [Google Scholar] [CrossRef]
- Wu, P.; Chen, D.; Ding, W.; Wu, P.; Hou, H.; Bai, Y.; Zhou, Y.; Li, K.; Xiang, S.; Liu, P.; et al. The trans-omics landscape of COVID-19. Nat. Commun. 2021, 12, 4543. [Google Scholar] [CrossRef]
- Atila, A.; Alay, H.; Yaman, M.E.; Akman, T.C.; Cadirci, E.; Bayrak, B.; Celik, S.; Atila, N.E.; Yaganoglu, A.M.; Kadioglu, Y.; et al. The serum amino acid profile in COVID-19. Amino Acids 2021, 53, 1569–1588. [Google Scholar] [CrossRef]
- Thomas, T.; Stefanoni, D.; Reisz, J.A.; Nemkov, T.; Bertolone, L.; Francis, R.O.; Hudson, K.E.; Zimring, J.C.; Hansen, K.C.; Hod, E.A.; et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight 2020, 5, e140327. [Google Scholar] [CrossRef]
- Masoodi, M.; Peschka, M.; Schmiedel, S.; Haddad, M.; Frye, M.; Maas, C.; Lohse, A.; Huber, S.; Kirchhof, P.; Nofer, J.-R.; et al. Disturbed lipid and amino acid metabolisms in COVID-19 patients. J. Mol. Med. 2022, 100, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Ansone, L.; Briviba, M.; Silamikelis, I.; Terentjeva, A.; Perkons, I.; Birzniece, L.; Rovite, V.; Rozentale, B.; Viksna, L.; Kolesova, O.; et al. Amino acid metabolism is significantly altered at the time of admission in hospital for severe COVID-19 patients: Findings from longitudinal targeted metabolomics analysis. Microbiol. Spectr. 2021, 9, e00338-21. [Google Scholar] [CrossRef] [PubMed]
- Dogan, H.O.; Senol, O.; Bolat, S.; Yildiz, S.N.; Buyuktuna, S.A.; Sariismailoglu, R.; Doğan, K.; Hasbek, M.; Hekim, S.N. Understanding the pathophysiologic changes via untargeted metabolomics in COVID-19 patients. J. Med. Virol. 2021, 93, 2340–2349. [Google Scholar] [CrossRef] [PubMed]
- Kimhofer, T.; Lodge, S.; Whiley, L.; Gray, N.; Loo, R.L.; Nawler, N.G.; Nitschke, P.; Bong, S.-H.; Morrison, D.L.; Begum, S.; et al. Integrative modeling of quantitative plasma lipoprotein, metabolic, and amino acid data reveals a multiorgan pathophysiological signature of SARS-CoV-2 infection. Proteome Res. 2020, 19, 4442–4454. [Google Scholar] [CrossRef]
- Lee, W.J.; Su, Y.; Baloni, P.; Chen, D.; Pavlovitch-Bedzyk, A.J.; Yuan, D.; Duvvuri, V.R.; Ng, R.H.; Choi, J.; Xie, J.; et al. Integrated analysis of plasma and single immune cells uncovers metabolic changes in individuals with COVID-19. Nat. Biotechnol. 2021, 40, 110–120. [Google Scholar] [CrossRef]
- Rees, C.A.; Rostade, C.A.; Mantus, G.; Anderson, E.J.; Chahroudi, A.; Jaggi, P.; Wrammert, J.; Ochoa, J.B.; Ochoa, A.; Basu, R.K.; et al. Altered amino acid profile in patients with SARS-CoV-2 infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2101708118. [Google Scholar] [CrossRef]
- Wang, H.; Yan, D.; Li, Y.; Gong, Y.; Mai, Y.; Li, B.; Zhu, X.; Wan, X.; Xie, L.; Jiang, H.; et al. Clinical and antibody signatures of severe and non-severe SARS-CoV-2 patients. Infect. Dis. Poverty 2022, 11, 15. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Thomas, T.; Akpan, I.J.; Reisz, J.A.; Cendali, F.I.; Gamboni, F.; Nemkov, T.; Thangaraju, K.; Katneni, U.; Tanaka, K.; et al. Biological and clinical factors contributing to the metabolic heterogeneity of hospitalized patients with and without COVID-19. Cells 2021, 10, 2293. [Google Scholar] [CrossRef]
- Aydin, H.; Tekin, Y.K.; Korkmaz, I.; Tekin, G.; Yurtbay, S.; Keles, S.; Hekim, N. Glutamine-driven metabolic adaptation to COVID-19 infection. Ind. J. Clin. Biochem. 2023, 38, 83–93. [Google Scholar] [CrossRef]
- Paez-Franco, J.C.; Maravillas-Montero, J.L.; Mejia-Dominguez, N.R.; Torres-Ruiz, J.; Tamez-Torres, K.M.; Perez-Fragoso, A.; Germán-Acacio, J.M.; Ponce-de-León, A.; Gómez-Martín, D.; Ulloa-Aguirre, A. Metabolomics analysis identifies glutamic acid and cysteine imbalances in COVID-19 patients without comorbid conditions. Implications on redox homeostasis and COVID-19. PLoS ONE 2022, 17, e0274910. [Google Scholar] [CrossRef]
- Li, X.-K.; Tu, B.; Zhang, X.-A.; Xu, W.; Chen, G.H.; Zhao, G.H.; Xu, B.; Zheng, J.; Yan, Y.; Hao, P.; et al. Dysregulation of glutamine/glutamate metabolism in COVID-19 patients: A metabolism study in African population and mini meta-analysis. J. Med. Virol. 2023, 95, e28150. [Google Scholar] [CrossRef]
- Kim, J.; Zhang, J.; Cha, Y.; Kolitz, S.; Funt, J.; Escalante, R.; Barrett, S.; Kusko, R.; Zeskind, B.; Kaufman, H. Advanced bioinformatics rapidly identifies existing therapeutics for patients with coronavirus disease-2019 (COVID-19). J. Transl. Med. 2020, 18, 257. [Google Scholar] [CrossRef]
- Li, M.; Wu, Y.; Ye, L. The role of amino acids in endothelial biology and function. Cells 2022, 11, 1372. [Google Scholar] [CrossRef]
- Wu, G.; Meininger, C.J. Arginine nutrition and cardiovascular function. J. Nutr. 2000, 130, 2626–2629. [Google Scholar] [CrossRef]
- Halaby, M.J.; McGaha, T.L. Amino acid transport and metabolism in myeloid function. Front. Immunol. 2021, 12, 695238. [Google Scholar] [CrossRef]
- Sikalidis, A.K. Amino acids and immune responses: A role for cysteine, glutamine, phenylalanine, tryptophan, and arginine in T-cell function and cancer. Pathol. Oncol. Res. 2015, 21, 9–17. [Google Scholar] [CrossRef]
- Miyajima, M. Amino acids: Key sources for immunometabolites and immunotransmitters. Int. Immunol. 2020, 32, 435–446. [Google Scholar] [CrossRef]
- Matsuyama, T.; Yoshinaga, S.K.; Shibue, K.; Mak, T.W. Comorbidity-associated glutamine deficiency is a predisposition to severe COVID-19. Cell Death Differ. 2021, 28, 3199–3213. [Google Scholar] [CrossRef]
- Xi, P.; Jiang, Z.; Zheng, C.; Lin, Y.; Wu, G. Regulation of protein metabolism by glutamine: Implications for nutrition and health. Front. Biosci. 2011, 16, 578–597. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The diverse functions of glutamine in metabolism, cell biology, and cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef]
- Sadaf, A.; Quinn, C.T. L-glutamine for sickle cell disease: Knight or pawn. Exp. Biol. Med. 2020, 245, 146–154. [Google Scholar] [CrossRef]
- Hirabara, S.M.; Gorjao, R.; Levada-Pires, A.C.; Masi, L.N.; Hatanaka, E.; Cury-Boaventura, M.F.; da Silva, E.B.; dos Santos-Oliveira, L.C.; Diniz, V.L.S.; Serdan, T.A.D.; et al. Host cell glutamine metabolism as a potential antiviral target. Clin. Sci. 2021, 135, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, S.; Singh, M.; Kirtipal, N.; Kang, S.G. SARS-CoV-2 and glutamine: SARS-CoV-2 triggered pathogenesis via metabolic reprogramming of glutamine in host cells. Front. Mol. Biosci. 2021, 7, 627842. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, M.; Uysal, B.B.; Ikitimur, H.; Ozcan, E.; Islamoglu, M.S.; Aktepe, E.; Yavuzer, H.; Yavuzer, S. Effect of oral L-glutamine supplementation on COVID-19 treatment. Clin. Nutr. Exp. 2020, 33, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.; Horriatkhah, E.; Mohajery, R. The effect of glutamine supplementation on serum levels of some inflammatory factors, oxidative stress, and appetite in COVID-19 patients: A case-control study. Inflammopharmacology 2021, 29, 1769–1776. [Google Scholar] [CrossRef]
- Soliman, O.M.; Thabet, A.M.A.; Abudahab, G.M.; Kamel, E.Z. The impact of glutamine supplementation on short-term mortality of COVID-19 diseased patients admitted to the ICU: A single-blind randomized clinical trial. Egypt. J. Anaesth. 2022, 38, 94–100. [Google Scholar] [CrossRef]
- Matthews, D.E.; Marano, M.A.; Campbell, R.G. Splanchnic bed utilization of glutamine and glutamic acid in humans. Am. J. Physiol. 1993, 264, E848–E854. [Google Scholar] [CrossRef]
- Cruzat, V.F.; Rogero, M.M.; Tirapegui, J. Effects of supplementation with free glutamine and the dipeptide alanyl-glutamine on parameters of muscle damage and inflammation in rats submitted to prolonged exercise. Cell Biochem. Funct. 2010, 28, 24–30. [Google Scholar] [CrossRef]
- Durante, W. Targeting arginine in COVID-19-induced immunopathology and vasculopathy. Metabolites 2022, 12, 240. [Google Scholar] [CrossRef]
- Adebayo, A.; Varzideh, F.; Wilson, S.; Gambardella, J.; Eacobacci, M.; Jankauskas, S.S.; Donkor, K.; Kansakar, U.; Trimarco, V.; Mone, P.; et al. L-Arginine and COVID-19: An update. Nutrients 2021, 13, 3951. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Cho, L.; Brennan, D.M.; Hazen, S.L. Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J. Am. Coll. Cardiol. 2009, 53, 2061–2067. [Google Scholar] [CrossRef]
- Heyland, D.; Muscedere, J.; Wischmeyer, P.E.; Cook, D.; Jones, G.; Albert, M.; Elke, G.; Berger, M.M.; Day, A.G.; The Canadian Critical Care Trials Group. A randomized trial of glutamine and antioxidants in critical ill patients. N. Engl. J. Med. 2013, 368, 1489–1497. [Google Scholar] [CrossRef]
- Eaton, M.D.; Scala, A.R. Inhibitory effect of glutamine and ammonia on replication of influenza virus in ascites tumor cells. Virology 1961, 13, 300–307. [Google Scholar] [CrossRef]
- Maratos-Flier, E.; Goodman, M.J.; Murray, A.H.; Kahn, C.R. Ammonium processing and cytotoxicity of reovirus, a nonenveloped virus. J. Clin. Investig. 1986, 78, 1003–1007. [Google Scholar] [CrossRef]
- Farias, G.; Navarrete, E.; Kiss, J.; Kuznar, J. Effect of ammonium chloride on the multiplication of infectious pancreatic necrosis virus. Arch. Virol. 1988, 98, 155–162. [Google Scholar] [CrossRef]
- Dabydeen, S.A.; Meneses, P.I. The role of NH4Cl and cysteine proteases in human papillomavirus type 16 infection. Virol. J. 2009, 6, 109. [Google Scholar] [CrossRef]
- Kheirabad, K.; Nourozi, M. Ammonium chloride as a potential candidate for the treatment and controlling of COVID-19. Iran. J. Virol. 2020, 14, 42–44. [Google Scholar]
- Fang, W.; Jiang, J.; Su, L.; Shu, T.; Liu, H.; Lai, S.; Ghiladi, R.A.; Wang, J. The role of NO in COVID-19 and potential therapeutic strategies. Free Radic. Biol. Med. 2021, 163, 153–162. [Google Scholar] [CrossRef]
- Rajendran, R.; Chathambath, A.; Al-Sehemi, A.G.; Pannipara, M.; Unnikrishnan, M.K.; Aleya, L.; Raghavan, R.P.; Mathew, B. Critical role of nitric oxide in impeding COVID-19 transmission and prevention.: A promising possibility. Environ. Sci. Pollut. Res. 2022, 29, 38657–38672. [Google Scholar] [CrossRef]
- Agarwal, S.; Kaur, S.; Asuru, T.R.; Joshi, G.; Shrimali, N.M.; Singh, A.; Singh, O.N.; Srivastva, P.; Shrivastava, T.; Vrati, S.; et al. Dietary alpha-ketoglutarate inhibits SARS-CoV-2 infection and rescues inflamed lungs to restore O2 saturation by inhibiting pAkt. Clin. Transl. Med. 2022, 12, e1041. [Google Scholar] [CrossRef]
- Tekwe, C.D.; Yao, K.; Lei, J.; Li, X.; Gupta, A.; Luan, Y.; Meininger, C.J.; Bazer, F.W.; Wu, G. Oral administration of α-ketoglutarate enhances nitric oxide synthesis by endothelial cells and whole-body insulin sensitivity in diet-induced obese rats. Exp. Biol. Med. 2019, 244, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Piagnerelli, M.; Van Meerhaeghe, A.; Boudjeltia, K.Z. Heme oxygenase-1 (HO-1) cytoprotective pathway: A potential treatment strategy against coronavirus disease 2019 (COVID-19)-induced cytokine storm syndrome. Med. Hypotheses 2020, 144, 110242. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Wasan, H.; Reeta, K.H. Heme oxygenase-1 modulation: A potential therapeutic target for COVID-19 and associated complications. Free Radic. Biol. Med. 2020, 161, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Wagener, F.A.D.T.G.; Pickkers, P.; Peterson, S.J.; Immenschuh, S.; Abraham, N.G. Targeting the heme-heme oxygenase system to prevent severe complications following COVID-19 infections. Antioxidants 2020, 9, 540. [Google Scholar] [CrossRef]
- Espinoza, J.A.; Gonzalez, P.A.; Kalergis, A.M. Modulation of antiviral immunity by heme oxygenase-1. Am. J. Pathol. 2017, 187, 487–493. [Google Scholar] [CrossRef]
- Ma, Z.; Pu, F.; Zhang, X.; Yan, Y.; Zhao, L.; Zhang, A.; Li, N.; Zhou, E.-M.; Xiao, S. Carbon monoxide and biliverdin suppress bovine viral diarrhoea virus replication. J. Gen. Virol. 2017, 98, 2982–2992. [Google Scholar] [CrossRef]
- Zhang, A.; Zhao, L.; Li, N.; Duan, H.; Liu, H.; Pu, F.; Zhang, G.; Zhou, E.M.; Xiao, S. Carbon monoxide inhibits porcine reproductive and respiratory syndrome virus replication by the cyclic GMP/protein kinase G and NF-κB signaling pathway. J. Virol. 2017, 91, e01866-16. [Google Scholar] [CrossRef]
- Liu, X.-M.; Peyton, K.J.; Durante, W. Physiological cyclic strain promotes endothelial cell survival via the induction of heme oxygenase-1. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1634–H1643. [Google Scholar] [CrossRef]
- Peyton, K.J.; Behnammanesh, G.; Durante, G.L.; Durante, W. Canagliflozin inhibits human endothelial cell inflammation through the induction of heme oxygenase-1. Int. J. Mol. Sci. 2022, 23, 8777. [Google Scholar] [CrossRef]
- Liu, X.M.; Durante, Z.E.; Peyton, K.J.; Durante, W. Heme oxygenase-derived bilirubin counteracts HIV protease inhibitor-mediated endothelial cell dysfunction. Free Radic. Biol. Med. 2016, 94, 218–229. [Google Scholar] [CrossRef]
- Peng, L.; Mundada, L.; Stomel, J.M.; Liu, J.J.; Sun, S.F.; Fay, W.P. Induction of heme oxygenase-1 expression inhibits platelet-dependent thrombosis. Antioxid. Redox Signal. 2004, 6, 729–735. [Google Scholar] [CrossRef]
- True, A.L.; Olive, M.; Boehm, M.; San, H.; Westrick, R.J.; Raghavachari, N.; Xu, X.; Lynn, E.G.; Sack, M.N.; Munson, P.J.; et al. Heme oxygenase-1 deficiency accelerates formation of arterial thrombosis through oxidative damage to the endothelium, which is rescued by inhaled carbon monoxide. Circ. Res. 2007, 101, 893–901. [Google Scholar] [CrossRef]
- Detsika, M.G.; Nikitopoulou, I.; Veroutis, D.; Vassiliou, A.G.; Jahaj, E.; Tsipilis, S.; Athanassiou, N.; Gakiopoulou, H.; Gorgoulis, V.G.; Dimopoulou, I.; et al. Increase of HO-1 expression in critically ill COVID-19 patients is associated with poor prognosis and outcome. Antioxidants 2022, 11, 1300. [Google Scholar] [CrossRef]
- de Lima, F.; Moraes, C.R.P.; Barbosa, M.S.; Bombassaro, B.; Palma, A.C.; Dertkigil, S.S.J.; Moretti, M.L.; Orsi, F.A.; Annichino-Bizzacchi, J.M.; Mansour, E.; et al. Association of heme oxygenase-1, hemopexin, and heme levels with markers of disease severity in COVID-19. Exp. Biol. Med. 2023. [Google Scholar] [CrossRef]
- Andreas, M.; Oeser, C.; Kainz, F.M.; Shabanian, S.; Aref, T.; Bilban, M.; Messner, B.; Heidtmann, J.; Laufer, G.; Kocher, A.; et al. Intravenous heme arginate induces HO-1 (heme oxygenase-1) in the human heart: Randomized, placebo-controlled, safety, and feasibility pharmacokinetic study. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2755–2762. [Google Scholar] [CrossRef]
- Bozza, M.T.; Jeney, V. Pro-inflammatory actions of heme and other hemoglobin-derived DAMPS. Front. Immunol. 2020, 11, 1323. [Google Scholar] [CrossRef]
- Pergola, P.E.; Raskin, P.; Toto, R.D.; Meyer, C.J.; Huff, J.W.; Grossman, E.B.; Krauth, M.; Ruiz, S.; Audhya, P.; Christ-Schmidt, H.; et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N. Engl. J. Med. 2011, 365, 327–336. [Google Scholar] [CrossRef]
- De Zeeuw, D.; Akizawa, T.; Audhya, P.; Bakris, G.L.; Chin, M.; Christ-Schmidt, H.; Goldsberry, A.; Houser, M.; Krauth, M.; Heerspiink, H.J.L.; et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Eng. J. Med. 2013, 369, 2492–2503. [Google Scholar] [CrossRef]
- Kanda, H.; Yamawaka, K. Bardoxolone methyl: Drug development for diabetic kidney disease. Clin. Exp. Nephrol. 2020, 24, 857–864. [Google Scholar] [CrossRef]
- Oh, C.J.; Park, S.; Kim, Y.J.; Kim, H.J.; Jeong, N.H.; Choi, Y.K.; Go, Y.; Park, K.G.; Lee, I.K. Dimethylfumurate attenuates restenosis after acute vascular injury by cell-specific and Nrf2-dependent mechanisms. Redox Biol. 2014, 2, 855–864. [Google Scholar] [CrossRef]
- Behnammanesh, G.; Durante, G.L.; Khanna, Y.P.; Peyton, K.J.; Durante, W. Canagliflozin inhibits vascular smooth muscle cell proliferation and migration: Role of heme oxygenase-1. Redox Biol. 2020, 32, 101527. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Canvas Program Collaborative Group; et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Peyton, K.J.; Reyna, S.V.; Chapman, G.B.; Ensenat, D.E.; Liu, X.M.; Wang, H.; Schafer, A.I.; Durante, W. Heme oxygenase-1-derived carbon monoxide is an autocrine inhibitor of vascular smooth muscle cell growth. Blood 2002, 99, 4443–4448. [Google Scholar] [CrossRef] [PubMed]
- Hopper, C.P.; Meinel, L.; Stieger, C.; Otterbein, L.E. Where is the clinical breakthrough of HO-1/carbon monoxide therapeutics. Curr. Pharm. Des. 2018, 24, 2264–2282. [Google Scholar] [CrossRef] [PubMed]
- Foresti, R.; Motterlini, R. Interaction of carbon monoxide with transition metals: Evolutionary insights into drug development. Curr. Drug Targets 2010, 11, 1595–1604. [Google Scholar] [CrossRef]
- Motterlini, R.; Foresti, R. Biological signaling by carbon monoxide and carbon monoxide-releasing molecules. Am. J. Physiol. Cell Physiol. 2017, 312, C302–C313. [Google Scholar] [CrossRef]
- Wang, D.; Viennois, E.; Ji, K.; Damera, K.; Draganov, A.; Zheng, Y.; Dai, C.; Merlin, D.; Wang, B. A click-and-release approach to CO prodrugs. Chem. Commun. 2014, 50, 1589015893. [Google Scholar] [CrossRef]
- Tulis, D.A.; Keswani, A.N.; Peyton, K.J.; Wang, H.; Schafer, A.I.; Durante, W. Local administration of carbon monoxide inhibits neointima formation in balloon-injured rat carotid arteries. Cell. Mol. Biol. 2005, 51, 441–446. [Google Scholar]
- Belcher, J.D.; Gomperts, E.; Nguyen, J.; Chen, C.; Abdulla, F.; Kiser, Z.M.; Gallo, D.; Levy, H.; Otterbein, L.E. Oral carbon monoxide therapy in murine sickle cell disease: Beneficial effects on vaso-occlusion, inflammation, and anemia. PLoS ONE 2018, 13, e0205194. [Google Scholar] [CrossRef]
- Ollinger, R.; Bilban, M.; Erat, A.; Froio, A.; McDaid, J.; Tyagi, S.; Csizmadia, E.; Graca-Souza, A.V.; Liloia, A.; Soares, M.P.; et al. Bilirubin: A natural inhibitor of vascular smooth muscle cell proliferation. Circulation 2005, 112, 1030–1039. [Google Scholar] [CrossRef]
- Nakao, A.; Murase, N.; Ho, C.; Toyokawa, H.; Billiar, T.R.; Kanno, S. Biliverdin administration prevents the formation of intimal hyperplasia induced by vascular injury. Circulation 2005, 112, 587–591. [Google Scholar] [CrossRef]
- Peyton, K.J.; Shebib, A.R.; Azam, M.A.; Liu, X.M.; Tulis, D.A.; Durante, W. Bilirubin inhibits neointima formation and vascular smooth muscle cell proliferation and migration. Front. Pharmacol. 2012, 3, 48. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, H.; Kang, S.; Lee, J.; Park, J.; Jon, S. Bilirubin nanoparticles as a nanomedicine for anti-inflammation. Angew. Chem. Int. Ed. 2016, 55, 7460–7463. [Google Scholar] [CrossRef]
- Zhang, D.; Chando, T.; Everett, D.W.; Patten, C.J.; Dehal, S.S.; Humphreys, G. In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of this property to in vivo bilirubin glucuronidation. Drug Metab. Dispos. 2005, 33, 1729–1739. [Google Scholar] [CrossRef]
- Pattanawongsa, A.; Chau, N.; Rowland, A.; Miner, J.O. Inhibition of human UDP-glucuronosyltransferase enzymes by canagliflozin and dapagliflozin: Implications for drug-drug interactions. Drug Metab. Dispos. 2015, 43, 1468–1476. [Google Scholar] [CrossRef]
- Dekker, D.; Dorresteijn, M.J.; Pijnenburg, M.; Heemskerk, S.; Rasing-Hoogveld, A.; Burger, D.M.; Wagener, F.A.D.T.G.; Smits, P. The bilirubin-increasing drug atazanavir improves endothelial function in patients with type 2 diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 458–463. [Google Scholar] [CrossRef]
- Durante, W.; Behnammanesh, G.; Peyton, K.J. Effects of sodium-glucose co-transporter 2 inhibitors on vascular cell function and arterial remodeling. Int. J. Mol. Sci. 2021, 22, 8786. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durante, W. Glutamine Deficiency Promotes Immune and Endothelial Cell Dysfunction in COVID-19. Int. J. Mol. Sci. 2023, 24, 7593. https://doi.org/10.3390/ijms24087593
Durante W. Glutamine Deficiency Promotes Immune and Endothelial Cell Dysfunction in COVID-19. International Journal of Molecular Sciences. 2023; 24(8):7593. https://doi.org/10.3390/ijms24087593
Chicago/Turabian StyleDurante, William. 2023. "Glutamine Deficiency Promotes Immune and Endothelial Cell Dysfunction in COVID-19" International Journal of Molecular Sciences 24, no. 8: 7593. https://doi.org/10.3390/ijms24087593