Altered Tryptophan-Kynurenine Pathway in Delirium: A Review of the Current Literature
Abstract
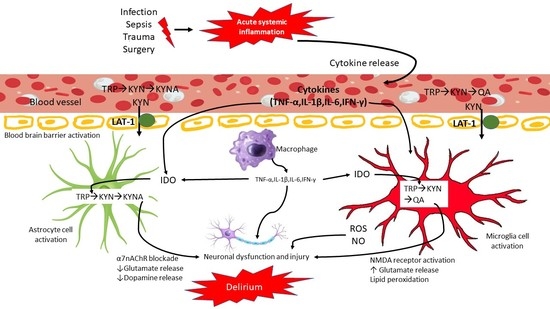
:1. Introduction
2. Tryptophan-Kynurenine Pathway
3. Role of the Tryptophan-Kynurenine Pathway in Delirium
3.1. Role of Indoleamine 2,3-Deoxygenase in Delirium
3.2. Role of Neuroactive KP Metabolites in Delirium
3.2.1. Quinolinic Acid
3.2.2. Kynurenic Acid
4. Translational Implications
5. Future Research Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Delirium Association; American Delirium Society. The DSM-5 criteria, level of arousal and delirium diagnosis: Inclusiveness is safer. BMC Med. 2014, 12, 141. [Google Scholar]
- Smit, L.; Wiegers, E.J.A.; Trogrlic, Z.; Rietdijk, W.J.R.; Gommers, D.; Ista, E.; van der Jagt, M. Prognostic significance of delirium subtypes in critically ill medical and surgical patients: A secondary analysis of a prospective multicenter study. J. Intensive Care 2022, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Salluh, J.I.F.; Wang, H.; Schneider, E.B.; Nagaraja, N.; Yenokyan, G.; Damluji, A.; Serafim, R.B.; Stevens, R.D. Outcome of delirium in critically ill patients: Systematic review and meta-analysis. BMJ 2015, 350, h2538. [Google Scholar] [CrossRef] [Green Version]
- Leslie, D.L.; Marcantonio, E.R.; Zhang, Y.; Leo-Summers, L.; Inouye, S.K. One-year health care costs associated with delirium in the elderly population. Arch. Intern. Med. 2008, 168, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, D.H.; Muniz-Terrera, G.; Keage, H.A.; Stephan, B.C.; Fleming, J.; Ince, P.G.; Matthews, F.E.; Cunningham, C.; Ely, E.W.; MacLullich, A.M. Association of delirium with cognitive decline in late life: A neuropathologic study of 3 population-based cohort studies. JAMA Psychiatry 2017, 74, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Witlox, J.; Eurelings, L.S.; de Jonghe, J.F.; Kalisvaart, K.J.; Eikelenboom, P.; Van Gool, W.A. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: A meta-analysis. JAMA 2010, 304, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Pandharipande, P.P.; Girard, T.D.; Ely, E.W. Long-term cognitive impairment after critical illness. N. Engl. J. Med. 2014, 370, 185–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, L.O.J.E.; Berning, M.J.; Stanich, J.A.; Gerberi, D.J.; Murad, M.H.; Han, J.H.; Bellolio, F. Risk Factors for Delirium in Older Adults in the Emergency Department: A Systematic Review and Meta-Analysis. Ann. Emerg. Med. 2021, 78, 549–565. [Google Scholar] [CrossRef]
- Mariz, J.; Costa Castanho, T.; Teixeira, J.; Sousa, N.; Correia Santos, N. Delirium diagnostic and screening instruments in the emergency department: An up-to-date systematic review. Geriatrics 2016, 1, 22. [Google Scholar] [CrossRef] [Green Version]
- Khor, H.M.; Ong, H.C.; Tan, B.K.; Low, C.M.; Saedon, N.I.; Tan, K.M.; Chin, A.V.; Kamaruzzaman, S.B.; Tan, M.P. Assessment of Delirium Using the Confusion Assessment Method in Older Adult Inpatients in Malaysia. Geriatrics 2019, 4, 52. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, J.E.S.L.; Berning, M.J.; Stanich, J.A.; Gerberi, D.J.; Han, J.; Bellolio, F. Risk factors for delirium among older adults in the emergency department: A systematic review protocol. BMJ Open 2020, 10, e039175. [Google Scholar] [CrossRef]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of brain aging: Adaptive and pathological modification by metabolic states. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef] [Green Version]
- Magny, E.; Le Petitcorps, H.; Pociumban, M.; Bouksani-Kacher, Z.; Pautas, É.; Belmin, J.; Bastuji-Garin, S.; Lafuente-Lafuente, C. Predisposing and precipitating factors for delirium in community-dwelling older adults admitted to hospital with this condition: A prospective case series. PLoS ONE 2018, 13, e0193034. [Google Scholar] [CrossRef]
- Jayaswal, A.K.; Sampath, H.; Soohinda, G.; Dutta, S. Delirium in medical intensive care units: Incidence, subtypes, risk factors, and outcome. Ind. J. Psychiatry 2019, 61, 352. [Google Scholar] [CrossRef]
- Cascella, M.; Muzio, M.R.; Bimonte, S.; Cuomo, A.; Jakobsson, J.G. Postoperative delirium and postoperative cognitive dysfunction: Updates in pathophysiology, potential translational approaches to clinical practice and further research perspectives. Minerva Anestesiol. 2018, 84, 246–260. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.; Dong, T.; Yang, Z.; Zhang, Q.; Zhang, Y. Risk factors for postoperative delirium following hip fracture repair in elderly patients: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2017, 29, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Evered, L.; Silbert, B.; Knopman, D.S.; Scott, D.A.; DeKosky, S.T.; Rasmussen, L.S.; Oh, E.S.; Crosby, G.; Berger, M.; Eckenhoff, R.G. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br. J. Anaesth. 2018, 121, 1005–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, C.; Wang, M.; Geng, Y.; Ke, J.; Dong, P.; Qin, J.; Zhong, D. Risk factors for postoperative delirium in ICU patients with severe illness based on systematic review and meta-analysis. Ann. Palliat. Med. 2022, 11, 309–320. [Google Scholar] [CrossRef]
- Bramley, P.; McArthur, K.; Blayney, A.; McCullagh, I. Risk factors for postoperative delirium: An umbrella review of systematic reviews. Int. J. Surg. 2021, 93, 106063. [Google Scholar] [CrossRef]
- Oh, E.S.; Fong, T.G.; Hshieh, T.T.; Inouye, S.K. Delirium in Older Persons: Advances in Diagnosis and Treatment. JAMA 2017, 318, 1161–1174. [Google Scholar] [CrossRef]
- Farrell, K.R.; Ganzini, L. Misdiagnosing delirium as depression in medically ill elderly patients. Arch. Intern. Med. 1995, 155, 2459–2464. [Google Scholar] [CrossRef] [PubMed]
- Hercus, C.; Hudaib, A.-R. Delirium misdiagnosis risk in psychiatry: A machine learning-logistic regression predictive algorithm. BMC Health Serv. Res. 2020, 20, 151. [Google Scholar] [CrossRef] [Green Version]
- Rosen, T.; Connors, S.; Clark, S.; Halpern, A.; Stern, M.E.; DeWald, J.; Lachs, M.S.; Flomenbaum, N. Assessment and Management of Delirium in Older Adults in the Emergency Department: Literature Review to Inform Development of a Novel Clinical Protocol. Adv. Emerg. Nurs. J. 2015, 37, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, J.R. Acute Brain Failure: Pathophysiology, Diagnosis, Management, and Sequelae of Delirium. Crit. Care Clin. 2017, 33, 461–519. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Chen, Z.; Leng, S.X. Connection between Systemic Inflammation and Neuroinflammation Underlies Neuroprotective Mechanism of Several Phytochemicals in Neurodegenerative Diseases. Oxid. Med. Cell Longev. 2018, 2018, 1972714. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Koyama, Y.; Shimada, S. Inflammation From Peripheral Organs to the Brain: How Does Systemic Inflammation Cause Neuroinflammation? Front. Aging Neurosci. 2022, 14, 903455. [Google Scholar] [CrossRef]
- Voils, S.A.; Shoulders, B.R.; Singh, S.; Solberg, L.M.; Garrett, T.J.; Frye, R.F. Intensive care unit delirium in surgical patients is associated with upregulation in tryptophan metabolism. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020, 40, 500–506. [Google Scholar] [CrossRef]
- Chiappelli, J.; Notarangelo, F.M.; Pocivavsek, A.; Thomas, M.A.; Rowland, L.M.; Schwarcz, R.; Hong, L.E. Influence of plasma cytokines on kynurenine and kynurenic acid in schizophrenia. Neuropsychopharmacology 2018, 43, 1675–1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. S2), 136–153. [Google Scholar] [CrossRef] [Green Version]
- Bistrian, B. Systemic response to inflammation. Nutr. Rev. 2007, 65, S170–S172. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, N.; Otten, W.; Merlot, E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids 2011, 41, 1195–1205. [Google Scholar] [CrossRef]
- Mithaiwala, M.N.; Santana-Coelho, D.; Porter, G.A.; O’Connor, J.C. Neuroinflammation and the Kynurenine Pathway in CNS Disease: Molecular Mechanisms and Therapeutic Implications. Cells 2021, 10, 1548. [Google Scholar] [CrossRef] [PubMed]
- Capece, L.; Lewis-Ballester, A.; Marti, M.A.; Estrin, D.A.; Yeh, S.-R. Molecular basis for the substrate stereoselectivity in tryptophan dioxygenase. Biochemistry 2011, 50, 10910–10918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, N.J.; Thomas, S.R. Molecules in focus: Indoleamine 2,3-dioxygenase. Int. J. Biochem. Cell Biol. 2007, 39, 2167–2172. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Guillemin, G.J.; Mansour, H.; Chan-Ling, T.; Grant, R. Changes in kynurenine pathway metabolism in the brain, liver and kidney of aged female Wistar rats. FEBS J. 2011, 278, 4425–4434. [Google Scholar] [CrossRef] [PubMed]
- Larkin, P.B.; Sathyasaikumar, K.V.; Notarangelo, F.M.; Funakoshi, H.; Nakamura, T.; Schwarcz, R.; Muchowski, P.J. Tryptophan 2,3-dioxygenase and indoleamine 2,3-dioxygenase 1 make separate, tissue-specific contributions to basal and inflammation-induced kynurenine pathway metabolism in mice. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2016, 1860, 2345–2354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lestage, J.; Verrier, D.; Palin, K.; Dantzer, R. The enzyme indoleamine 2, 3-dioxygenase is induced in the mouse brain in response to peripheral administration of lipopolysaccharide and superantigen. Brain Behav. Immun. 2002, 16, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Comai, S.; Costa, C.V.; Ragazzi, E.; Bertazzo, A.; Allegri, G. The effect of age on the enzyme activities of tryptophan metabolism along the kynurenine pathway in rats. Clin. Chim. Acta 2005, 360, 67–80. [Google Scholar] [CrossRef]
- Amori, L.; Guidetti, P.; Pellicciari, R.; Kajii, Y.; Schwarcz, R. On the relationship between the two branches of the kynurenine pathway in the rat brain in vivo. J. Neurochem. 2009, 109, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Walsh, H.A.; Botting, N.P. Purification and biochemical characterization of some of the properties of recombinant human kynureninase. Eur. J. Biochem. 2002, 269, 2069–2074. [Google Scholar] [CrossRef]
- Badawy, A.A.-B. Hypothesis kynurenic and quinolinic acids: The main players of the kynurenine pathway and opponents in inflammatory disease. Med. Hypotheses 2018, 118, 129–138. [Google Scholar] [CrossRef] [PubMed]
- MacLullich, A.M.; Ferguson, K.J.; Miller, T.; de Rooij, S.E.; Cunningham, C. Unravelling the pathophysiology of delirium: A focus on the role of aberrant stress responses. J. Psychosom. Res. 2008, 65, 229–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dostal, C.R.; Gamsby, N.S.; Lawson, M.A.; McCusker, R.H. Glia- and tissue-specific changes in the Kynurenine Pathway after treatment of mice with lipopolysaccharide and dexamethasone. Brain Behav. Immun. 2018, 69, 321–335. [Google Scholar] [CrossRef]
- Dostal, C.R.; Sulzer, M.C.; Kelley, K.W.; Freund, G.G.; McCusker, R.H. Glial and tissue-specific regulation of Kynurenine Pathway dioxygenases by acute stress of mice. Neurobiol. Stress 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Maes, M.; Rief, W. Diagnostic classifications in depression and somatization should include biomarkers, such as disorders in the tryptophan catabolite (TRYCAT) pathway. Psychiatry Res. 2012, 196, 243–249. [Google Scholar] [CrossRef]
- Sekine, A.; Kuroki, Y.; Urata, T.; Mori, N.; Fukuwatari, T. Inhibition of large neutral amino acid transporters suppresses kynurenic acid production via inhibition of kynurenine uptake in rodent brain. Neurochem. Res. 2016, 41, 2256–2266. [Google Scholar] [CrossRef]
- Walker, A.K.; Wing, E.E.; Banks, W.A.; Dantzer, R. Leucine competes with kynurenine for blood-to-brain transport and prevents lipopolysaccharide-induced depression-like behavior in mice. Mol. Psychiatry 2019, 24, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, G.J.; Kerr, S.J.; Smythe, G.A.; Smith, D.G.; Kapoor, V.; Armati, P.J.; Croitoru, J.; Brew, B.J. Kynurenine pathway metabolism in human astrocytes: A paradox for neuronal protection. J Neurochem. 2001, 78, 842–853. [Google Scholar] [CrossRef]
- Garrison, A.M.; Parrott, J.M.; Tuñon, A.; Delgado, J.; Redus, L.; O’Connor, J.C. Kynurenine pathway metabolic balance influences microglia activity: Targeting kynurenine monooxygenase to dampen neuroinflammation. Psychoneuroendocrinology 2018, 94, 1–10. [Google Scholar] [CrossRef]
- Westhoff, D.; Engelen-Lee, J.Y.; Hoogland, I.C.M.; Aronica, E.M.A.; van Westerloo, D.J.; van de Beek, D.; van Gool, W.A. Systemic infection and microglia activation: A prospective postmortem study in sepsis patients. Immun. Ageing 2019, 16, 18. [Google Scholar] [CrossRef] [Green Version]
- Lemstra, A.W.; Groen in’t Woud, J.C.; Hoozemans, J.J.; van Haastert, E.S.; Rozemuller, A.J.; Eikelenboom, P.; van Gool, W.A. Microglia activation in sepsis: A case-control study. J. Neuroinflamm. 2007, 4, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munster, B.C.v.; Aronica, E.; Zwinderman, A.H.; Eikelenboom, P.; Cunningham, C.; Rooij, S.E.J.A.d. Neuroinflammation in delirium: A postmortem case-control study. Rejuvenation Res. 2011, 14, 615–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, R.; Kan, M.-Q.; Wang, S.-G.; Yang, R.-H.; Zhang, S.-G. Disrupted tryptophan metabolism induced cognitive impairment in a mouse model of sepsis-associated encephalopathy. Inflammation 2016, 39, 550–560. [Google Scholar] [CrossRef]
- Heisler, J.M.; O’Connor, J.C. Indoleamine 2,3-dioxygenase-dependent neurotoxic kynurenine metabolism mediates inflammation-induced deficit in recognition memory. Brain Behav. Immun. 2015, 50, 115–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoogland, I.; Westhoff, D.; Engelen-Lee, J.-Y.; Melief, J.; Valls Serón, M.; Houben-Weerts, J.H.; Huitinga, I.; Van Westerloo, D.J.; van der Poll, T.; van Gool, W.A. Microglial activation after systemic stimulation with lipopolysaccharide and Escherichia coli. Front. Cell. Neurosci. 2018, 12, 110. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.R.A.; Morandi, A.; Girard, T.D.; Thompson, J.L.; Boomershine, C.S.; Shintani, A.K.; Ely, E.W.; Pandharipande, P.P. The association of the kynurenine pathway of tryptophan metabolism with acute brain dysfunction during critical illness. Crit. Care Med. 2012, 40, 835. [Google Scholar] [CrossRef] [Green Version]
- Darcy, C.J.; Davis, J.S.; Woodberry, T.; McNeil, Y.R.; Stephens, D.P.; Yeo, T.W.; Anstey, N.M. An observational cohort study of the kynurenine to tryptophan ratio in sepsis: Association with impaired immune and microvascular function. PLoS ONE 2011, 6, e21185. [Google Scholar] [CrossRef] [Green Version]
- Nettis, M.A.; Veronese, M.; Nikkheslat, N.; Mariani, N.; Lombardo, G.; Sforzini, L.; Enache, D.; Harrison, N.A.; Turkheimer, F.E.; Mondelli, V.; et al. PET imaging shows no changes in TSPO brain density after IFN-α immune challenge in healthy human volunteers. Transl. Psychiatry 2020, 10, 89. [Google Scholar] [CrossRef] [Green Version]
- Egberts, A.; Fekkes, D.; Ziere, G.; Van der Cammen, T.J.M.; Mattace-Raso, F.U.S. Potential Influence of Aspirin on Neopterin and Tryptophan Levels in Patients with a Delirium. Geriatrics 2016, 1, 10. [Google Scholar] [CrossRef] [Green Version]
- de Jonghe, A.; van Munster, B.C.; Fekkes, D.; van Oosten, H.E.; de Rooij, S.E. The tryptophan depletion theory in delirium: Not confirmed in elderly hip fracture patients. Psychosomatics 2012, 53, 236–243. [Google Scholar] [CrossRef]
- Watne, L.O.; Pollmann, C.T.; Neerland, B.E.; Quist-Paulsen, E.; Halaas, N.B.; Idland, A.-V.; Hassel, B.; Henjum, K.; Knapskog, A.-B.; Frihagen, F. Cerebrospinal fluid quinolinic acid is strongly associated with delirium and mortality in hip fracture patients. J. Clin. Investig. 2022, 133, e163472. [Google Scholar] [CrossRef]
- Tripp, B.A.; Dillon, S.T.; Yuan, M.; Asara, J.M.; Vasunilashorn, S.M.; Fong, T.G.; Metzger, E.D.; Inouye, S.K.; Xie, Z.; Ngo, L.H.; et al. Targeted metabolomics analysis of postoperative delirium. Sci. Rep. 2021, 11, 1521. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Hu, J.; Ma, D. Postoperative delirium: Perioperative assessment, risk reduction, and management. Br. J. Anaesth. 2020, 125, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.N.; Raeburn, C.D.; Angles, E.M.; Moss, M. Low tryptophan levels are associated with postoperative delirium in the elderly. Am. J. Surg. 2008, 196, 670–674. [Google Scholar] [CrossRef] [Green Version]
- van der Mast, R.C.; van den Broek, W.W.; Fekkes, D.; Pepplinkhuizen, L.; Habbema, J.D. Is delirium after cardiac surgery related to plasma amino acids and physical condition? J. Neuropsychiatry Clin. Neurosci. 2000, 12, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Robinson, T.N.; Dunn, C.L.; Adams, J.C.; Hawkins, C.L.; Tran, Z.V.; Raeburn, C.D.; Moss, M. Tryptophan supplementation and postoperative delirium--a randomized controlled trial. J. Am. Geriatr. Soc. 2014, 62, 1764–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Gomes, M.G.; Souza, L.C.; Goes, A.R.; Del Fabbro, L.; Filho, C.B.; Donato, F.; Prigol, M.; Luchese, C.; Roman, S.S.; Puntel, R.L.; et al. Fish oil ameliorates sickness behavior induced by lipopolysaccharide in aged mice through the modulation of kynurenine pathway. J. Nutr. Biochem. 2018, 58, 37–48. [Google Scholar] [CrossRef]
- Agostini, A.; Yuchun, D.; Li, B.; Kendall, D.A.; Pardon, M.C. Sex-specific hippocampal metabolic signatures at the onset of systemic inflammation with lipopolysaccharide in the APPswe/PS1dE9 mouse model of Alzheimer’s disease. Brain Behav. Immun. 2020, 83, 87–111. [Google Scholar] [CrossRef]
- de Gomes, M.G.; Teixeira, F.E.G.; de Carvalho, F.B.; Pacheco, C.O.; da Silva Neto, M.R.; Giacomeli, R.; Ramalho, J.B.; Dos Santos, R.B.; Domingues, W.B.; Campos, V.F.; et al. Curcumin-loaded lipid-core nanocapsules attenuates the immune challenge LPS-induced in rats: Neuroinflammatory and behavioral response in sickness behavior. J. Neuroimmunol. 2020, 345, 577270. [Google Scholar] [CrossRef]
- Tufvesson-Alm, M.; Imbeault, S.; Liu, X.C.; Zheng, Y.; Faka, A.; Choi, D.S.; Schwieler, L.; Engberg, G.; Erhardt, S. Repeated administration of LPS exaggerates amphetamine-induced locomotor response and causes learning deficits in mice. J. Neuroimmunol. 2020, 349, 577401. [Google Scholar] [CrossRef]
- Kang, A.; Hao, H.; Zheng, X.; Liang, Y.; Xie, Y.; Xie, T.; Dai, C.; Zhao, Q.; Wu, X.; Xie, L.; et al. Peripheral anti-inflammatory effects explain the ginsenosides paradox between poor brain distribution and anti-depression efficacy. J. Neuroinflamm. 2011, 8, 100. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Cai, H.; Chen, L.; Liang, D.; Yang, R.; Dang, R.; Jiang, P. Quantitative profiling of neurotransmitter abnormalities in the hippocampus of rats treated with lipopolysaccharide: Focusing on kynurenine pathway and implications for depression. J. Neuroimmunol. 2016, 295–296, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Golia, M.T.; Poggini, S.; Alboni, S.; Garofalo, S.; Albanese, N.C.; Viglione, A.; Ajmone-Cat, M.A.; St-Pierre, A.; Brunello, N.; Limatola, C. Interplay between inflammation and neural plasticity: Both immune activation and suppression impair LTP and BDNF expression. Brain Behav. Immun. 2019, 81, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.K.; Budac, D.P.; Bisulco, S.; Lee, A.W.; Smith, R.A.; Beenders, B.; Kelley, K.W.; Dantzer, R. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology 2013, 38, 1609–1616. [Google Scholar] [CrossRef]
- Zhao, X.; Cao, F.; Liu, Q.; Li, X.; Xu, G.; Liu, G.; Zhang, Y.; Yang, X.; Yi, S.; Xu, F.; et al. Behavioral, inflammatory and neurochemical disturbances in LPS and UCMS-induced mouse models of depression. Behav. Brain Res. 2019, 364, 494–502. [Google Scholar] [CrossRef]
- Schneiders, J.; Fuchs, F.; Damm, J.; Herden, C.; Gerstberger, R.; Soares, D.M.; Roth, J.; Rummel, C. The transcription factor nuclear factor interleukin 6 mediates pro- and anti-inflammatory responses during LPS-induced systemic inflammation in mice. Brain Behav. Immun. 2015, 48, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Dinel, A.L.; André, C.; Aubert, A.; Ferreira, G.; Layé, S.; Castanon, N. Lipopolysaccharide-induced brain activation of the indoleamine 2,3-dioxygenase and depressive-like behavior are impaired in a mouse model of metabolic syndrome. Psychoneuroendocrinology 2014, 40, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Gao, R.; Zhang, Y.; Jiang, N.; Chen, Y.; Sun, J.; Wang, Q.; Fan, B.; Liu, X.; Wang, F. S-equol, a metabolite of dietary soy isoflavones, alleviates lipopolysaccharide-induced depressive-like behavior in mice by inhibiting neuroinflammation and enhancing synaptic plasticity. Food Funct. 2021, 12, 5770–5778. [Google Scholar] [CrossRef]
- Farzi, A.; Reichmann, F.; Meinitzer, A.; Mayerhofer, R.; Jain, P.; Hassan, A.M.; Fröhlich, E.E.; Wagner, K.; Painsipp, E.; Rinner, B.; et al. Synergistic effects of NOD1 or NOD2 and TLR4 activation on mouse sickness behavior in relation to immune and brain activity markers. Brain Behav. Immun. 2015, 44, 106–120. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Fan, K.; Liu, Y.; Liu, G.; Yang, X.; Ma, J. Cathepsin C Aggravates Neuroinflammation Involved in Disturbances of Behaviour and Neurochemistry in Acute and Chronic Stress-Induced Murine Model of Depression. Neurochem. Res. 2018, 43, 89–100. [Google Scholar] [CrossRef]
- Carabelli, B.; Delattre, A.M.; Waltrick, A.P.F.; Araújo, G.; Suchecki, D.; Machado, R.B.; de Souza, L.E.R.; Zanata, S.M.; Zanoveli, J.M.; Ferraz, A.C. Fish-oil supplementation decreases Indoleamine-2,3-Dioxygenase expression and increases hippocampal serotonin levels in the LPS depression model. Behav. Brain Res. 2020, 390, 112675. [Google Scholar] [CrossRef] [PubMed]
- Peyton, L.; Oliveros, A.; Tufvesson-Alm, M.; Schwieler, L.; Starski, P.; Engberg, G.; Erhardt, S.; Choi, D.S. Lipopolysaccharide Increases Cortical Kynurenic Acid and Deficits in Reference Memory in Mice. Int. J. Tryptophan Res. 2019, 12, 1178646919891169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imbeault, S.; Goiny, M.; Liu, X.; Erhardt, S. Effects of IDO1 and TDO2 inhibition on cognitive deficits and anxiety following LPS-induced neuroinflammation. Acta Neuropsychiatr. 2020, 32, 43–53. [Google Scholar] [CrossRef]
- Choubey, P.; Kwatra, M.; Pandey, S.N.; Kumar, D.; Dwivedi, D.K.; Rajput, P.; Mishra, A.; Lahkar, M.; Jangra, A. Ameliorative effect of fisetin against lipopolysaccharide and restraint stress-induced behavioral deficits via modulation of NF-κB and IDO-1. Psychopharmacology 2019, 236, 741–752. [Google Scholar] [CrossRef]
- Comim, C.M.; Freiberger, V.; Ventura, L.; Mina, F.; Ferreira, G.K.; Michels, M.; Generoso, J.S.; Streck, E.L.; Quevedo, J.; Barichello, T. Inhibition of indoleamine 2, 3-dioxygenase 1/2 prevented cognitive impairment and energetic metabolism changes in the hippocampus of adult rats subjected to polymicrobial sepsis. J. Neuroimmunol. 2017, 305, 167–171. [Google Scholar] [CrossRef]
- Danielski, L.G.; Giustina, A.D.; Goldim, M.P.; Florentino, D.; Mathias, K.; Garbossa, L.; de Bona Schraiber, R.; Laurentino, A.O.M.; Goulart, M.; Michels, M.; et al. Vitamin B(6) Reduces Neurochemical and Long-Term Cognitive Alterations After Polymicrobial Sepsis: Involvement of the Kynurenine Pathway Modulation. Mol Neurobiol. 2018, 55, 5255–5268. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, L.; Tang, L.; Liu, F.; Chen, Z.; Zhang, J.; Chen, L.; Pang, C.; Yu, X. Role of the indoleamine-2, 3-dioxygenase/kynurenine pathway of tryptophan metabolism in behavioral alterations in a hepatic encephalopathy rat model. J. Neuroinflamm. 2018, 15, 3. [Google Scholar] [CrossRef] [Green Version]
- Gibney, S.M.; McGuinness, B.; Prendergast, C.; Harkin, A.; Connor, T.J. Poly I: C-induced activation of the immune response is accompanied by depression and anxiety-like behaviours, kynurenine pathway activation and reduced BDNF expression. Brain Behav. Immun. 2013, 28, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.; Griffin, É.W.; O’Loughlin, E.; Lyons, A.; Sherwin, E.; Ahmed, S.; Stevenson, N.J.; Harkin, A.; Cunningham, C. Interdependent and independent roles of type I interferons and IL-6 in innate immune, neuroinflammatory and sickness behaviour responses to systemic poly I: C. Brain Behav. Immun. 2015, 48, 274–286. [Google Scholar] [CrossRef] [Green Version]
- Cathomas, F.; Fuertig, R.; Sigrist, H.; Newman, G.N.; Hoop, V.; Bizzozzero, M.; Mueller, A.; Luippold, A.; Ceci, A.; Hengerer, B. CD40-TNF activation in mice induces extended sickness behavior syndrome co-incident with but not dependent on activation of the kynurenine pathway. Brain Behav. Immun. 2015, 50, 125–140. [Google Scholar] [CrossRef] [Green Version]
- Kelley, K.W.; O’Connor, J.C.; Lawson, M.A.; Dantzer, R.; Rodriguez-Zas, S.L.; McCusker, R.H. Aging leads to prolonged duration of inflammation-induced depression-like behavior caused by Bacillus Calmette-Guerin. Brain Behav. Immun. 2013, 32, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, E.S.; Akeju, O.; Avidan, M.S.; Cunningham, C.; Hayden, K.M.; Jones, R.N.; Khachaturian, A.S.; Khan, B.A.; Marcantonio, E.R.; Needham, D.M. A roadmap to advance delirium research: Recommendations from the NIDUS Scientific Think Tank. Alzheimer’s Dement. 2020, 16, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.H.; Skelly, D.T.; Murray, C.; Hennessy, E.; Bowen, J.; Norton, S.; Brayne, C.; Rahkonen, T.; Sulkava, R.; Sanderson, D.J. Worsening cognitive impairment and neurodegenerative pathology progressively increase risk for delirium. Am. J. Geriatr. Psychiatry 2015, 23, 403–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Bi, W.; Xiao, S.; Lan, X.; Cheng, X.; Zhang, J.; Lu, D.; Wei, W.; Wang, Y.; Li, H. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 2019, 9, 5790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirthgen, E.; Tuchscherer, M.; Otten, W.; Domanska, G.; Wollenhaupt, K.; Tuchscherer, A.; Kanitz, E. Activation of indoleamine 2,3-dioxygenase by LPS in a porcine model. Innate Immun. 2014, 20, 30–39. [Google Scholar] [CrossRef]
- Larsson, M.K.; Faka, A.; Bhat, M.; Imbeault, S.; Goiny, M.; Orhan, F.; Oliveros, A.; Ståhl, S.; Liu, X.C.; Choi, D.S.; et al. Repeated LPS Injection Induces Distinct Changes in the Kynurenine Pathway in Mice. Neurochem. Res. 2016, 41, 2243–2255. [Google Scholar] [CrossRef]
- Zhai, L.; Bell, A.; Ladomersky, E.; Lauing, K.L.; Bollu, L.; Sosman, J.A.; Zhang, B.; Wu, J.D.; Miller, S.D.; Meeks, J.J.; et al. Immunosuppressive IDO in Cancer: Mechanisms of Action, Animal Models, and Targeting Strategies. Front. Immunol. 2020, 11, 1185. [Google Scholar] [CrossRef]
- Ogbechi, J.; Clanchy, F.I.; Huang, Y.S.; Topping, L.M.; Stone, T.W.; Williams, R.O. IDO activation, inflammation and musculoskeletal disease. Exp. Gerontol. 2020, 131, 110820. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Schurink, B.; Roos, E.; Nossent, E.J.; Duitman, J.W.; Vlaar, A.P.; van der Valk, P.; Vaz, F.M.; Yeh, S.R.; Geeraerts, Z.; et al. Indoleamine 2,3-dioxygenase (IDO)-1 and IDO-2 activity and severe course of COVID-19. J. Pathol. 2022, 256, 256–261. [Google Scholar] [CrossRef]
- Hoshi, M.; Osawa, Y.; Ito, H.; Ohtaki, H.; Ando, T.; Takamatsu, M.; Hara, A.; Saito, K.; Seishima, M. Blockade of Indoleamine 2,3-Dioxygenase Reduces Mortality from Peritonitis and Sepsis in Mice by Regulating Functions of CD11b+ Peritoneal Cells. Infect. Immun. 2014, 82, 4487–4495. [Google Scholar] [CrossRef] [Green Version]
- Wirthgen, E.; Otten, W.; Tuchscherer, M.; Tuchscherer, A.; Domanska, G.; Brenmoehl, J.; Günther, J.; Ohde, D.; Weitschies, W.; Seidlitz, A.; et al. Effects of 1-Methyltryptophan on Immune Responses and the Kynurenine Pathway after Lipopolysaccharide Challenge in Pigs. Int. J. Mol. Sci. 2018, 19, 3009. [Google Scholar] [CrossRef] [Green Version]
- Pérez-De La Cruz, V.; Carrillo-Mora, P.; Santamaría, A. Quinolinic Acid, an endogenous molecule combining excitotoxicity, oxidative stress and other toxic mechanisms. Int. J. Tryptophan Res. IJTR 2012, 5, 1–8. [Google Scholar] [CrossRef]
- Lugo-Huitrón, R.; Ugalde Muñiz, P.; Pineda, B.; Pedraza-Chaverrí, J.; Ríos, C.; Pérez-de la Cruz, V. Quinolinic acid: An endogenous neurotoxin with multiple targets. Oxid. Med. Cell Longev. 2013, 2013, 104024. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Guillemin, G.J. Kynurenine pathway metabolites in humans: Disease and healthy states. Int. J. Tryptophan Res. 2009, 2, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Matyja, E. Intracellular calcium overload in a model of quinolinic acid neurotoxicity in organotypic culture of rat hippocampus; inhibited by nimodipine. Folia Neuropathol. 1997, 35, 8–17. [Google Scholar]
- Duncan, R.S.; Goad, D.L.; Grillo, M.A.; Kaja, S.; Payne, A.J.; Koulen, P. Control of intracellular calcium signaling as a neuroprotective strategy. Molecules 2010, 15, 1168–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosoi, R.; Fujii, Y.; Hiroyuki, O.; Shukuri, M.; Nishiyama, S.; Kanazawa, M.; Todoroki, K.; Arano, Y.; Sakai, T.; Tsukada, H.; et al. Evaluation of intracellular processes in quinolinic acid-induced brain damage by imaging reactive oxygen species generation and mitochondrial complex I activity. EJNMMI Res. 2021, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.C.; Miller, L.P.; Oldendorf, W.H.; Schwarcz, R. Studies on the disposition of quinolinic acid after intracerebral or systemic administration in the rat. Exp. Neurol. 1984, 84, 428–440. [Google Scholar] [CrossRef]
- Kubicova, L.; Hadacek, F.; Chobot, V. Quinolinic acid: Neurotoxin or oxidative stress modulator? Int. J. Mol. Sci. 2013, 14, 21328–21338. [Google Scholar] [CrossRef]
- Mallard, A.R.; Spathis, J.G.; Coombes, J.S. Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and exercise. Free Radic. Biol. Med. 2020, 160, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Merry, T.L.; Ristow, M. Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and the anti-oxidant response in mice. J. Physiol. 2016, 594, 5195–5207. [Google Scholar] [CrossRef] [PubMed]
- Velagapudi, R.; El-Bakoush, A.; Olajide, O.A. Activation of Nrf2 Pathway Contributes to Neuroprotection by the Dietary Flavonoid Tiliroside. Mol. Neurobiol. 2018, 55, 8103–8123. [Google Scholar] [CrossRef] [Green Version]
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Haque, M.N.; Moon, I.S. Neuroprotection Against Oxidative Stress: Phytochemicals Targeting TrkB Signaling and the Nrf2-ARE Antioxidant System. Front. Mol. Neurosci. 2020, 13, 116. [Google Scholar] [CrossRef]
- Dodson, M.; Anandhan, A.; Zhang, D.D.; Madhavan, L. An NRF2 Perspective on Stem Cells and Ageing. Front. Aging 2021, 2, 686. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, A.; Jiménez-Capdeville, M.E.; Camacho, A.; Rodríguez-Martínez, E.; Flores, A.; Galván-Arzate, S. In vivo hydroxyl radical formation after quinolinic acid infusion into rat corpus striatum. Neuroreport 2001, 12, 2693–2696. [Google Scholar] [CrossRef]
- Santamaría, A.; Salvatierra-Sánchez, R.; Vázquez-Román, B.; Santiago-López, D.; Villeda-Hernández, J.; Galván-Arzate, S.; Jiménez-Capdeville, M.E.; Ali, S.F. Protective effects of the antioxidant selenium on quinolinic acid-induced neurotoxicity in rats: In vitro and in vivo studies. J. Neurochem. 2003, 86, 479–488. [Google Scholar] [CrossRef]
- Davis, I.; Yang, Y.; Wherritt, D.; Liu, A. Reassignment of the human aldehyde dehydrogenase ALDH8A1 (ALDH12) to the kynurenine pathway in tryptophan catabolism. J. Biol. Chem. 2018, 293, 9594–9603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwahashi, H.; Kawamori, H.; Fukushima, K. Quinolinic acid, α-picolinic acid, fusaric acid, and 2, 6-pyridinedicarboxylic acid enhance the Fenton reaction in phosphate buffer. Chem.-Biol. Interact. 1999, 118, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Št’astný, F.; Lisý, V.; Mareš, V.; Lisá, V.; Balcar, V.J.; Santamaría, A. Quinolinic acid induces NMDA receptor-mediated lipid peroxidation in rat brain microvessels. Redox Rep. 2004, 9, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Toufekoula, C.; Papadakis, V.; Tsaganos, T.; Routsi, C.; Orfanos, S.E.; Kotanidou, A.; Carrer, D.P.; Raftogiannis, M.; Baziaka, F.; Giamarellos-Bourboulis, E.J. Compartmentalization of lipid peroxidation in sepsis by multidrug-resistant gram-negative bacteria: Experimental and clinical evidence. Crit. Care 2013, 17, R6. [Google Scholar] [CrossRef] [Green Version]
- Robson, M.J.A.; Alston, R.P.; Andrews, P.J.D.; Souter, M.J. S100β after coronary artery surgery: Association with lipid peroxidation and neurocognitive scores. Crit. Care 2000, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- van der Mast, R.C. Pathophysiology of delirium. J. Geriatr. Psychiatry Neurol. 1998, 11, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Twomey, E.C.; Sobolevsky, A.I. Structural Mechanisms of Gating in Ionotropic Glutamate Receptors. Biochemistry 2018, 57, 267–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdonk, F.; Petit, A.C.; Abdel-Ahad, P.; Vinckier, F.; Jouvion, G.; de Maricourt, P.; De Medeiros, G.F.; Danckaert, A.; Van Steenwinckel, J.; Blatzer, M.; et al. Microglial production of quinolinic acid as a target and a biomarker of the antidepressant effect of ketamine. Brain Behav. Immun. 2019, 81, 361–373. [Google Scholar] [CrossRef]
- Hollinger, A.; Rüst, C.A.; Riegger, H.; Gysi, B.; Tran, F.; Brügger, J.; Huber, J.; Toft, K.; Surbeck, M.; Schmid, H.R.; et al. Ketamine vs. haloperidol for prevention of cognitive dysfunction and postoperative delirium: A phase IV multicentre randomised placebo-controlled double-blind clinical trial. J. Clin. Anesth. 2021, 68, 110099. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.S.; Schmitz, F.; Marques, E.P.; Siebert, C.; Wyse, A.T.S. Intrastriatal Quinolinic Acid Administration Impairs Redox Homeostasis and Induces Inflammatory Changes: Prevention by Kynurenic Acid. Neurotox. Res. 2020, 38, 50–58. [Google Scholar] [CrossRef]
- Poles, M.Z.; Nászai, A.; Gulácsi, L.; Czakó, B.L.; Gál, K.G.; Glenz, R.J.; Dookhun, D.; Rutai, A.; Tallósy, S.P.; Szabó, A.; et al. Kynurenic Acid and Its Synthetic Derivatives Protect Against Sepsis-Associated Neutrophil Activation and Brain Mitochondrial Dysfunction in Rats. Front. Immunol. 2021, 12, 717157. [Google Scholar] [CrossRef]
- Wang, G.; Cao, K.; Liu, K.; Xue, Y.; Roberts, A.I.; Li, F.; Han, Y.; Rabson, A.B.; Wang, Y.; Shi, Y. Kynurenic acid, an IDO metabolite, controls TSG-6-mediatedimmunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018, 25, 1209–1223. [Google Scholar] [CrossRef] [Green Version]
- García-Lara, L.; Pérez-Severiano, F.; González-Esquivel, D.; Elizondo, G.; Segovia, J. Absence of aryl hydrocarbon receptors increases endogenous kynurenic acid levels and protects mouse brain against excitotoxic insult and oxidative stress. J. Neurosci. Res. 2015, 93, 1423–1433. [Google Scholar] [CrossRef]
- DiNatale, B.C.; Murray, I.A.; Schroeder, J.C.; Flaveny, C.A.; Lahoti, T.S.; Laurenzana, E.M.; Omiecinski, C.J.; Perdew, G.H. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol. Sci. 2010, 115, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Martos, D.; Tuka, B.; Tanaka, M.; Vécsei, L.; Telegdy, G. Memory Enhancement with Kynurenic Acid and Its Mechanisms in Neurotransmission. Biomedicines 2022, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Collier, M.E.; Zhang, S.; Scrutton, N.S.; Giorgini, F. Inflammation control and improvement of cognitive function in COVID-19 infections: Is there a role for kynurenine 3-monooxygenase inhibition? Drug Discov. Today 2021, 26, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Chess, A.C.; Simoni, M.K.; Alling, T.E.; Bucci, D.J. Elevations of Endogenous Kynurenic Acid Produce Spatial Working Memory Deficits. Schizophr. Bull. 2006, 33, 797–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klausing, A.D.; Fukuwatari, T.; Bucci, D.J.; Schwarcz, R. Stress-induced impairment in fear discrimination is causally related to increased kynurenic acid formation in the prefrontal cortex. Psychopharmacology 2020, 237, 1931–1941. [Google Scholar] [CrossRef]
- Dobelis, P.; Staley, K.J.; Cooper, D.C. Lack of modulation of nicotinic acetylcholine alpha-7 receptor currents by kynurenic acid in adult hippocampal interneurons. PLoS ONE 2012, 7, e41108. [Google Scholar] [CrossRef] [Green Version]
- Mok, M.S.; Fricker, A.-C.; Weil, A.; Kew, J.N. Electrophysiological characterisation of the actions of kynurenic acid at ligand-gated ion channels. Neuropharmacology 2009, 57, 242–249. [Google Scholar] [CrossRef]
- Stone, T.W. Kynurenic acid blocks nicotinic synaptic transmission to hippocampal interneurons in young rats. Eur. J. Neurosci. 2007, 25, 2656–2665. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Lu, Y.; Geng, Y.; Lu, W.; Chen, Y. How could N-Methyl-D-Aspartate Receptor Antagonists Lead to Excitation Instead of Inhibition? Brain Sci. Adv. 2018, 4, 73–98. [Google Scholar] [CrossRef]
- Kozak, R.; Campbell, B.M.; Strick, C.A.; Horner, W.; Hoffmann, W.E.; Kiss, T.; Chapin, D.S.; McGinnis, D.; Abbott, A.L.; Roberts, B.M.; et al. Reduction of brain kynurenic acid improves cognitive function. J. Neurosci. 2014, 34, 10592–10602. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Lindroth, H.; Chan, C.; Greene, R.; Serrano-Andrews, P.; Khan, S.; Rios, G.; Jabbari, S.; Lim, J.; Saykin, A.J.; et al. A Systematic Review of Delirium Biomarkers and Their Alignment with the NIA-AA Research Framework. J. Am. Geriatr. Soc. 2021, 69, 255–263. [Google Scholar] [CrossRef]
- Westbrook, R.; Chung, T.; Lovett, J.; Ward, C.; Joca, H.; Yang, H.; Khadeer, M.; Tian, J.; Xue, Q.L.; Le, A.; et al. Kynurenines link chronic inflammation to functional decline and physical frailty. JCI Insight 2020, 5, e136091. [Google Scholar] [CrossRef] [PubMed]
- Loretz, N.; Becker, C.; Hochstrasser, S.; Metzger, K.; Beck, K.; Mueller, J.; Gross, S.; Vincent, A.; Amacher, S.A.; Sutter, R.; et al. Activation of the kynurenine pathway predicts mortality and neurological outcome in cardiac arrest patients: A validation study. J. Crit. Care 2022, 67, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Wu, Y.-H.; Song, Y.; Yu, B. Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. J. Hematol. Oncol. 2021, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Zwilling, D.; Huang, S.Y.; Sathyasaikumar, K.V.; Notarangelo, F.M.; Guidetti, P.; Wu, H.Q.; Lee, J.; Truong, J.; Andrews-Zwilling, Y.; Hsieh, E.W.; et al. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell 2011, 145, 863–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Systemic Inflammation | Neuroinflammation | |
|---|---|---|
| Location | Throughout the body | Within the central nervous system (CNS) and spinal cord |
| Onset | Acute and chronic | Widely regarded as chronic |
| Innate immune cells | Macrophages, neutrophils, dendritic cells | Microglia and astrocytes |
| Causes | Trauma, sepsis, surgery | Autoimmune disease, metabolic disorders, aging, peripheral sources |
| Symptoms | Pain, increased temperature, redness, swelling | Memory deficit, depression, frailty |
| Associated conditions | Acute inflammation, such as trauma and infection Chronic inflammation, such as systemic lupus erythematosus and multiple sclerosis | Alzheimer’s disease and Parkinson’s disease |
| Author/Year | Study Design | Type of Sample/Metabolites Measured | Sample Size/Population Details | Delirium Assessment Tool | Age-Matched (Y/N) | Findings |
|---|---|---|---|---|---|---|
| Wilson et al. (2012) [56] | Observational | Plasma (TRP, KYN) | 84 (71 with brain dysfunction, 13 no brain dysfunction) in ICU | CAM | Y |
|
| Voils et al. (2020) [27] | Case-control | Plasma (TRP and KYN from the targeted metabolomics analysis) | 130 (65 patients with delirium, 65 control) in ICU | CAM-ICU | Y |
|
| Jonghe et al. (2012) [60] | Prospective cohort | Plasma (TRP, KYN) | 140 (71 with delirium, 69 without delirium) hip fracture patients | CAM | Y |
|
| Watne et al. (2022) [61] | Observational | Plasma and CSF (TRP, and KYN metabolites from the targeted metabolomics analysis) | 450 (224 with delirium, 226 without delirium) hip fracture patients | DSM-V criteria | Y |
|
| Egberts et al. (2016) [59] | Cross-sectional | Plasma (TRP) | 80 (22 had delirium, 58 without delirium) elderly in internal medicine and geriatric wards. | DSM-IV criteria | Y |
|
| Tripp et al. (2021) [62] | Case-control | Plasma (TRP and KYN metabolites from the targeted metabolomics analysis) | 104 (52 with delirium, 52 control) older adults undergoing major non-cardiac surgery | CAM | Y |
|
| Author | Type of Exposure | Type of Subjects | Test Battery | Follow up Duration | Findings |
|---|---|---|---|---|---|
| Comim et al., 2017 [85] | Sepsis induced by cecal ligation and perforation (CLP) | Wistar rat (60 days old) | Open field test (OFT) | 24 h and day 10 |
|
| Jiang et al., 2018 [87] | Laparotomy + bile duct ligation (BDL) | Male Wistar rats (220–240 g) | Sucrose preference test, forced swimming, marble burying test, and elevated plus maze | 0, 7, 14, 21, and 28 days |
|
| Cathomas et al., 2015 [90] | Single i.p. CD40 agonist antibody (CD40AB) injection | Male C57BL/6J mice (10–14-week-old) | Saccharine consumption, fear conditioning, and locomotor activity | Day 2, 4, 5, 6, 7, 8, and 12 after CD40AB injection |
|
| Gomes et al., 2018 [67] | Single i.p. LPS 0.33 mg/kg | Male C57BJ/6J mice (24-month-old) | Open field test, and sucrose preference test | 24 h |
|
| Agostini et al., 2020 [68] | Single intravenous LPS 100 μg/kg | Male and female APPswe/PS1dE9 mice model of Alzheimer’s disease and WT (4.5-month-old) | Food burrowing, and Y-maze test | 24 h, 48 h, and 96 h |
|
| Gomes et al., 2020 [69] | Single i.p. LPS 1mg/kg | Male Wistar rats (3-month-old) | Locomotor activity, rotarod test, and forced swimming test | 24 h |
|
| Tufvesson-Alm et al., 2020 [70] | Two doses i.p LPS 0.83 mg/kg 16 h apart | FVB/N and C57BL/6J mice (3–4-month-old) | Open field test, fear conditioning, and Y-maze | 24 and 48 h |
|
| Kang et al., 2011 [71] | Single i.p. LPS 0.8 mg/kg | CD-1 mice 10–12-week-old | Open field test, sucrose preference test, and forced swimming test | 24 h |
|
| Heisler & O’Connor 2015 [54] | i.p. LPS 0.5 mg/kg | C57BL/6J mice 12–16-week-old (group into wild type, IDO−/−, KMO transgenic mice) | Novel object recognition (NOR) | 24 to 48 h |
|
| Guo et al., 2016 [72] | Single i.p. LPS 3mg/kg Vs repeated i.p LPS 500 μg/kg every other day for 2 weeks | Sprague Dawley rats, 230–280 g | Sucrose preference test and forced swimming test | 2, 4, 6,8, and 10 h |
|
| Murray et al., 2015 [89] | i.p. Poly I:C (12 mg/kg) ± IL-6 (50 μg/kg) | Female C57BL6/J mice (WT And IFNAR1−/−) | Open field test and food burrowing | 24 h to 96 h |
|
| Gao et al., 2016 [53] | Cecal ligation and perforation (CLP) induced animal model of sepsis | Male C57BL/6 mice | Open field test and fear conditioning test | 12 h and day 14 |
|
| Golia et al., 2019 [73] | i.p LPS 0.33 and 0.83 mg/kg | Male C57BL/6 mice 12–15-week-old | Not specified | 3 h |
|
| Gibney et al., 2013 [88] | i.p. Poly I:C 6mg/kg | Male Sprague Dawley 250–350 g | Home cage activity test, saccharin preference, and open field test | 6, 2-, 48, and 72 h |
|
| Kelley et al., 2013 [91] | i.p. Bacillus Calmette–Guerin (BCG) i.p. 108 CFU/mice | Adult mice Balb/c 4–6-month-old vs. aged mice 20–24-month-old | Locomotor activity, sucrose preference test, and tail suspension test | 1, 7, 14 or 21 days |
|
| Walker et al., 2013 [74] | LPS i.p 1mg/kg (dose to induced depressive-like behavior) LPS i.p. 0.83 mg/kg (dose to induce acute sickness response) | CD-1 (6-week-old) C57BL/6J (12-week-old) | Locomotor activity, sucrose preference test, and forced swimming test | 2–28 h |
|
| Zhao et al., 2019 [75] | i.p LPS 0.5 mg/kg and/or unpredictable chronic mild stress (UCMS) | Adult male C57BL/6J mice, 8–12-week-old | Open field test, forced swimming test, and tail suspension test. | 24 h |
|
| Schneiders et al., 2015 [76] | i.p LPS 50 or 2500 μg/kg | C/EBPβ+/+ (Wild type) and C/EBPβ-/- (KO) mice 6–12 weeks old | Locomotor activity (two-compartment cage) | 8 or 24 h |
|
| Dinel et al., 2014 [77] | i.p LPS 5ug | Male db/db and db/+ mice between 10 and 12 weeks | Two-compartment cage and forced swimming test | 2 h to 25 h |
|
| Lu et al., 2021 [78] | i.p LPS 1.0 mg/kg | Male C57BL/6J mice, 4–5 weeks of age | Sucrose preference test, forced swimming test, and tail suspension test | 24 h |
|
| Farzi et al., 2015 [79] | i.p. LPS (0.1 or 0.83 mg/kg) | Male C57BL/6N mice, aged at 10 weeks | Sucrose preference test, open field test, forced swimming test, and tail suspension test | 21 h |
|
| Zhang et al., 2018 [80] | i.p. LPS (0.5 mg/kg) and/or unpredictable chronic mild stress (UCMS) | Cat C overexpression (CAT C OE) and Cat C knockdown (CAT C KD) transgenic mice together with wild type (WT) at 8-weeks-old | Open field test, forced swimming test, and tail suspension test | 24 h |
|
| Carabelli et al., 2020 [81] | i.p. LPS 250μg/kg | Male Wistar rats, 60 days-old | Open field test and forced swimming test | 24 h |
|
| Peyton et al., 2019 [82] | i.p. 2X LPS (0.25 mg/kg, 0.50mg/kg, or saline) 16 h apart | Male C57Bl/6J mice, three months old | Pavlovian conditioning, accelerated rotarod radial 8-arm maze, and prepulse inhibition | 24 h, 48 h, and 144 h |
|
| Danielski et al., 2018 [86] | Cecal ligation and perforation (CLP) | Male Wistar rats, approximately 60 days old, 250–350 g | Open field test and object recognition test | 24 h-day 10 |
|
| Imbeault et al., 2020 [83] | Single i.p LPS 0.83mg/kg | Male C57Bl6/NCrl mice aged 13 to 18 weeks | Elevated plus maze and fear conditioning test | 24–96 h |
|
| Choubey et al., 2019 [84] | Single i.p. LPS 0.83 mg/kg and/or restraint stress (RS) | Adult male Swiss albino mice | Open field test and forced swimming test | 3–24 h |
|
| Biomarker | Sample Type | Main Effects |
|---|---|---|
| CRP | Plasma |
|
| S-100B | Plasma |
|
| NSE | Plasma |
|
| Systemic inflammatory marker | Plasma |
|
| Neuroimaging | MRI/EEG |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phing, A.H.; Makpol, S.; Nasaruddin, M.L.; Wan Zaidi, W.A.; Ahmad, N.S.; Embong, H. Altered Tryptophan-Kynurenine Pathway in Delirium: A Review of the Current Literature. Int. J. Mol. Sci. 2023, 24, 5580. https://doi.org/10.3390/ijms24065580
Phing AH, Makpol S, Nasaruddin ML, Wan Zaidi WA, Ahmad NS, Embong H. Altered Tryptophan-Kynurenine Pathway in Delirium: A Review of the Current Literature. International Journal of Molecular Sciences. 2023; 24(6):5580. https://doi.org/10.3390/ijms24065580
Chicago/Turabian StylePhing, Ang Hui, Suzana Makpol, Muhammad Luqman Nasaruddin, Wan Asyraf Wan Zaidi, Nurul Saadah Ahmad, and Hashim Embong. 2023. "Altered Tryptophan-Kynurenine Pathway in Delirium: A Review of the Current Literature" International Journal of Molecular Sciences 24, no. 6: 5580. https://doi.org/10.3390/ijms24065580