An Insight into the Essential Role of Carbohydrate-Binding Modules in Enzymolysis of Xanthan
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sequence and Structure Comparison of PspCBM84 and MiCBMx
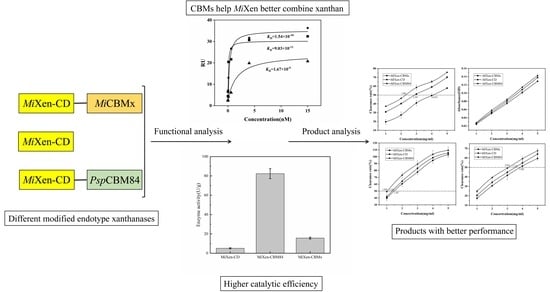
2.2. Construction and Characterization of Different Recombinant Forms of MiXen
2.3. Xanthanase Fused with PspCBM84 Exhibits Better Hydrolysis Performance against Xanthan
2.4. Xanthan Digests Prepared by MiXen-CBM84 Showed Improved Antioxidant Activity
3. Materials and Methods
3.1. Sequence Analysis and Structural Alignment of Enzymes
3.2. Strains, Plasmids and Culture Conditions
3.3. Cloning, Expression and Purification of Endotype Xanthanase Constructs
3.4. Enzyme Activity Assays
3.5. Biochemical Characteristics of the Recombinant Endotype Xanthanases
3.6. Surface Plasmon Resonance
3.7. Gel Permeation Chromatography
3.8. AFM Imaging of Xanthan Samples
3.9. Liquid Chromatography Analysis
3.10. UPLC-QTOF-MS Analysis
3.11. Antioxidant Activity Analysis of Xanthan Digests
3.12. Infrared Spectroscopy
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Becker, A.; Katzen, F.; Pühler, A.; Lelpi, L. Xanthan gum biosynthesis and application: A biochemical/genetic perspective. Appl. Microbiol. Biotechnol. 1998, 50, 145–152. [Google Scholar] [CrossRef]
- Patel, J.; Maji, B.; Moorthy, N.; Maiti, S. Xanthan gum derivatives: Review of synthesis, properties and diverse applications. RSC Adv. 2020, 10, 27103–27136. [Google Scholar] [CrossRef] [PubMed]
- Steffens, T.; Vorhölter, F.J.; Teckentrup, J.; Hublik, G.; Walhorn, V.; Anselmetti, D.; Puhler, A.; Niehaus, K.; Ortseifen, V. Two flagellar mutants of Xanthomonas campestris are characterized by enhanced xanthan production and higher xanthan viscosity. J. Biotechnol. 2022, 347, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.E.; Kenne, L.; Lindberg, B. Structure of the extracellular polysaccharide from Xanthomonas campestris. Carbohydr. Res. 1975, 45, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Nankai, H.; Hashimoto, W.; Murata, K. Molecular identification of family 38 α-mannosidase of Bacillus sp. strain GL1, responsible for complete depolymerization of xanthan. Appl. Environ. Microb. 2002, 68, 2731–2736. [Google Scholar] [CrossRef] [Green Version]
- Riaz, T.; Iqbal, M.W.; Jiang, B.; Chen, J.J. A review of the enzymatic, physical, and chemical modification techniques of xanthan gum. Int. J. Biol. Macromol. 2021, 186, 472–489. [Google Scholar] [CrossRef]
- Hashimoto, W.; Miki, H.; Tsuchiya, N.; Nankai, H.; Murata, K. Xanthan lyase of Bacillus sp. strain GL1 liberates pyruvylated mannose from xanthan side chains. Appl. Environ. Microb. 1998, 64, 3765–3768. [Google Scholar] [CrossRef] [Green Version]
- Sutherland, I.W. Xanthomonas polysaccharides—Improved methods for their comparison. Carbohydr. Polym. 1981, 1, 107–115. [Google Scholar] [CrossRef]
- Xiong, X.; Li, M.; Xie, J.; Jin, Q.; Xue, B.; Sun, T. Antioxidant activity of xanthan oligosaccharides prepared by different degradation methods. Carbohydr. Polym. 2013, 92, 1166–1171. [Google Scholar] [CrossRef]
- Xiong, X.; Li, M.; Xie, J.; Xue, B.; Sun, T. Preparation and antioxidant activity of xanthan oligosaccharides derivatives with similar substituting degrees. Food Chem. 2014, 164, 7–11. [Google Scholar] [CrossRef]
- Ahlgren, J.A. Characterization of Xanthan Gum Degrading Enzymes from a Heat-stable, Salt-tolerant Bacterial consortium. Pet. Sci. 1993, 39, 55–63. [Google Scholar]
- Ruijssenaars, J.A.; Jan, A.M.; Hartmans, S. A pyruvated mannose-specific xanthan lyase involved in xanthan degradation by Paenibacillus. Appl. Environ. Microb. 1999, 65, 2446–2452. [Google Scholar] [CrossRef] [Green Version]
- Moroz, O.V.; Jensen, P.F.; Mcdonald, S.P.; Mcgregor, N.; Wilson, K.S. Structural dynamics and catalytic properties of a multi-modular xanthanase. ACS Catal. 2018, 8, 6021–6034. [Google Scholar] [CrossRef]
- Yang, F.; Li, H.; Sun, J.; Guo, X.; Zhang, X. Characterization of a novel endo-type xanthanase MiXen from xanthan-degrading Microbacterium sp. XT11. Appl. Environ. Microb. 2018, 85, 1–47. [Google Scholar]
- Kool, M.M.; Schols, H.A.; Delahaije, R.J.; Sworn, G.; Wierenga, P.A.; Gruppen, H. The influence of the primary and secondary xanthan structure on the enzymatic hydrolysis of the xanthan backbone. Carbohydr. Polym. 2013, 97, 368–375. [Google Scholar] [CrossRef]
- Gu, J.; Wang, D.; Wang, Q.; Liu, W.; Chen, X.; Li, X.; Yang, F. Novel β-Glucosidase Mibgl3 from Microbacterium sp. XT11 with Oligoxanthan-Hydrolyzing Activity. J. Agric. Food. Chem. 2022, 70, 8713–8724. [Google Scholar] [CrossRef]
- Guillén, D.; Sánchez, S.; Rodríguez-Sanoja, R. Carbohydrate-binding domains: Multiplicity of biological roles. Appl. Microbiol Biotechnol. 2010, 85, 1241–1249. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Bao, C.; Dong, B.; Cao, Y. Characterization of a novel GH10 xylanase with a carbohydrate binding module from Aspergillus sulphureus and its synergistic hydrolysis activity with cellulase. Int. J. Biol. Macromol. 2021, 182, 701–711. [Google Scholar] [CrossRef]
- Deng, J.J.; Zhang, M.S.; Li, Z.W.; Lu, D.L.; Mao, H.H.; Zhu, M.J.; Luo, X.C. One-step processing of shrimp shell waste with a chitinase fused to a carbohydrate-binding module. Green Chem. 2020, 22, 6862–6873. [Google Scholar] [CrossRef]
- Zhou, J.; Gu, Q.; Shen, Y.; Harindintwali, J.; Yang, W.; Zou, S.; Han, M.; Ma, C.; Yu, X.; Liu, X. Enhancement of the performance of the GH75 family chitosanases by fusing a carbohydrate binding module and insights into their substrate binding mechanisms. LWT 2022, 163, 113390. [Google Scholar] [CrossRef]
- Zhou, J.; Harindintwali, J.D.; Yang, W.; Han, M.; Deng, B.; Luan, H.; Zhang, W.; Liu, X.; Yu, X. Engineering of a chitosanase fused to a carbohydrate-binding module for continuous production of desirable chitooligosaccharides. Carbohydr. Polym. 2021, 273, 118609. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, Q.; Wu, Y.; Sun, D.; Zhu, J.; Liu, C.; Liu, W. Carbohydrate-binding modules of ChiB and ChiC promote the chitinolytic system of Serratia marcescens BWL1001. Enzyme Microb. Technol. 2023, 162, 110118. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, A.; Pellegrini, V.O.A.; Curtolo, F.; Camilo, C.M.; Mello, B.L.; Johns, M.A.; Scott, J.L.; Guimaraes, F.E.C.; Polikarpov, I. Carbohydrate binding modules enhance cellulose enzymatic hydrolysis by increasing access of cellulases to the substrate. Carbohydr. Polym. 2019, 211, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Park, B.R.; Lee, H.N.; Jang, D.E.; Kang, H.J.; Ameer, K.; Kim, S.J.; Kim, S.J. Carbohydrate-binding module of cycloisomaltooligosaccharide glucanotransferase from Thermoanaerobacter thermocopriae improves its cyclodextran production. Enzym. Microb. Technol. 2022, 157, 110023. [Google Scholar] [CrossRef]
- Li, Y.; Song, W.; Yin, X.; Rao, S.; Zhang, Q.; Zhou, J.; Li, J.; Du, G.; Liu, S. Enhanced catalytic performance of thermophilic GH11 xylanase by fusing carbohydrate-binding module 9-2 and linker for better synergistic degradation of wheat bran. Process Biochem. 2022, 121, 349–359. [Google Scholar] [CrossRef]
- Hu, Y.; Li, H.; Ran, Q.; Liu, J.; Zhou, S.; Qiao, Q.; Song, H.; Peng, F.; Jiang, Z. Effect of carbohydrate binding modules alterations on catalytic activity of glycoside hydrolase family 6 exoglucanase from Chaetomium thermophilum to cellulose. Int. J. Biol. Macromol. 2021, 191, 222–229. [Google Scholar] [CrossRef]
- Bolam, D.N.; Ciruela, A.; Mcqueen-Mason, S.; Simpson, P.; Williamson, M.P.; Rixon, J.E.; Boraston, A.; Hazlewood, G.P.; Gilbert, H.J. Pseudomonas cellulose-binding domains mediate their effects by increasing enzyme substrate proximity. Biochem. J. 1998, 331, 775–781. [Google Scholar] [CrossRef]
- Liu, S.; Din, S. Replacement of carbohydrate binding modules improves acetyl xylan esterase activity and its synergistic hydrolysis of different substrates with xylanase. BMC Biotechnol. 2016, 16, 73. [Google Scholar] [CrossRef] [Green Version]
- Cadmus, M.C.; Slodki, M.E.; Nicholson, J.J. High-temperature, salt-tolerant xanthanase. J. Ind. Microbiol. 1989, 4, 127–133. [Google Scholar] [CrossRef]
- Hu, X.; Wang, K.; Yu, M.; He, P.; Qiao, H.; Zhang, H.; Wang, Z. Characterization and antioxidant activity of a low-molecular-weight xanthan gum. Biomolecules 2019, 730, 730. [Google Scholar] [CrossRef] [Green Version]
- Reva, B.A.; Finkelstein, A.V.; Skolnick, J. What is the probability of a chance prediction of a protein structure with an rmsd of 6 A? Fold Des. 1998, 3, 141–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Y.; Xu, J.; Fu, X.; Tan, M.; Liu, F.; Zheng, H.; Song, H. Effects of different carbohydrate-binding modules on the enzymatic properties of pullulanase. Int. J. Biol. Macromol. 2019, 137, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Coolbear, T.; Whittaker, J.M.; Daniel, R.M. The effect of metal ions on the activity and thermostability of the extracellular proteinase from a thermophilic Bacillus, strain EA.1. Biochem. J. 1992, 287, 367–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manjeet, K.; Madhuprakash, J.; Mormann, M.; Moerschbacher, B.M.; Podile, A.R. A carbohydrate binding module-5 is essential for oxidative cleavage of chitin by a multi-modular lytic polysaccharide monooxygenase from Bacillus thuringiensis serovar kurstaki. Int. J. Biol. Macromol. 2019, 127, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, C.; Du, X.; Peng, H.; Liu, L.; Xiao, Y.; He, C. Specific hydrolysis of curdlan with a novel glycoside hydrolase family 128 β-1, 3-endoglucanase containing a carbohydrate-binding module. Carbohydr. Polym. 2021, 253, 117276. [Google Scholar] [CrossRef] [PubMed]
- Sulyman, A.O.; Igunnu, A.; Malomo, S.O. Isolation, purification and characterization of cellulase produced by Aspergillus niger cultured on Arachis hypogaea shells. Heliyon 2020, 6, e05668. [Google Scholar] [CrossRef]
- Nisar, K.; Abdullah, R.; Kaleem, A.; Lqtedar, M.; Aftab, M.; Saleem, F. Purification, characterization and thermodynamic analysis of cellulases produced from Thermomyces dupontii and its industrial applications. Saudi J. Biol. Sci. 2022, 29, 103483. [Google Scholar] [CrossRef]
- Sriariyanun, M.; Tantayotai, P.; Yasurin, P.; Pornwongthong, P.; Cheenkachorn, K. Production, purification and characterization of an ionic liquid tolerant cellulase from Bacillus sp. isolated from rice paddy field soil. Electron. J. Biotechnol. 2016, 19, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Sajjad, M.; Sadaf, S.; Zafar, R.; Niazi, U.; Akhtar, M. The nature of the carbohydrate binding module determines the catalytic efficiency of xylanase Z of Clostridium thermocellum. J. Biotechnol. 2013, 168, 403–408. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, R.; Zeng, J.; Li, G.; Li, X. Characterization of destrins with different dextrose equivalents. Molecules 2010, 15, 5162–5173. [Google Scholar] [CrossRef] [Green Version]
- Hung, Y.-H.R.; Chen, G.-W.; Pan, C.-L.; Lin, H.-T.V. Production of ulvan oligosaccharides with antioxidant and angiotensin-converting enzyme-inhibitory activities by microbial enzymatic hydrolysis. Fermentation 2021, 7, 160. [Google Scholar] [CrossRef]
- Varma, S.D.; Hegde, K.; Henein, M. Oxidative damage to mouse lens in culture. Protective effect of pyruvate. BBA Gen. Subj. 2003, 1621, 246–252. [Google Scholar] [CrossRef]
- Unger, T.; Jacobovitch, Y.; Dantes, A.; Bernheim, R.; Peleg, Y. Applications of the Restriction Free (RF) cloning procedure for molecular manipulations and protein expression. J. Struct. Biol. 2010, 172, 34–44. [Google Scholar] [CrossRef]
- Xie, X.; Tian, Y.; Tian, J.; Ning, W.; Wang, C. Construction of T7 RNA polymerase gene expression system in Anabaena sp. PCC 7120 for the expression of hG-CSF. Chin. J. Biotechnol. 2020, 36, 2467–2477. [Google Scholar]
- Mitchell, D.M.; Gennis, R.B. Rapid purification of wildtype and mutant cytochrome c oxidase from Rhodobacter sphaeroides by Ni(2+)-NTA affinity chromatography. FEBS Lett. 1995, 368, 148. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Behera, P.K.; Madhuprakash, J. Efficient conversion of crystalline chitin to N-acetylglucosamine and N,N′-diacetylchitobiose by the enzyme cocktail produced by Paenibacillus sp. LS1. Carbohydr. Polym. 2020, 250, 116889. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, L.; Bao, M.; Liu, Z.; Yu, W.; Han, F. Functional characterization of carbohydrate-binding modules in a new alginate lyase, TsAly7B, from Thalassomonas sp. LD5. Mar. Drugs 2019, 18, 25. [Google Scholar] [CrossRef] [Green Version]
- Guan, F.; Han, Y.; Yan, K.; Zhang, Y.; Zhang, Z.; Wu, N.; Tian, J. Highly efficient production of chitooligosaccharides by enzymes mined directly from the marine metagenome. Carbohydr. Polym. 2020, 234, 115909. [Google Scholar] [CrossRef]
- Hashimoto, W.; Nankai, H.; Mikami, B.; Murata, K. Crystal structure of Bacillus sp. GL1 xanthan lyase, which acts on the side chains of xanthan. J. Biol. Chem. 2003, 278, 7663–7673. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, T.; Takamura, H.; Matoba, T.; Terao, J. HPLC Method for Evaluation of the Free Radical-scavenging Activity of Foods by Using 1,1-Diphenyl-2-picrylhydrazyl. Biosci. Biotechnol. Biochem. 1998, 62, 1201–1204. [Google Scholar] [CrossRef]
- Guo, Z.; Xing, R.; Liu, S.; Yu, H.; Wang, P.; Li, C.; Li, P. The synthesis and antioxidant activity of the Schiff bases of chitosan and carboxymethyl chitosan. Bioorg. Med. Chem. Lett. 2005, 15, 4600–4603. [Google Scholar] [CrossRef] [PubMed]
- Gow, C.Y.; Hui, Y.C. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food. Chem. 1994, 43, 27–32. [Google Scholar]
- Chan, E.; Lim, Y.Y.; Chew, Y.L. Antioxidant activity of Camellia sinensis leaves and tea from a lowland plantation in Malaysia. Food Chem. 2007, 102, 1214–1222. [Google Scholar] [CrossRef]









| Enzyme | Alpha Helix | Extended Strand | Beta Turn | Random Coil |
|---|---|---|---|---|
| PspCBM84 | 11.35% | 34.04% | 8.51% | 46.1% |
| MiCBMx | 6.71% | 34.90% | 9.40% | 48.99% |
| Reagents Added | Relative Activity (MiXen-CD) | Relative Activity (MiXen-CBM84) | Relative Activity (MiXen-CBMx) |
|---|---|---|---|
| None | 100 | 100 | 100 |
| K+ | 70.7 ± 8.29 | 96.3 ± 2.29 | 114.1 ± 1.64 |
| Fe2+ | 76.3 ± 5.45 | 126.8 ± 5.26 | 76.3 ± 6.86 |
| Fe3+ | 123.8 ± 8.66 | 81.4 ± 3.45 | 87.8 ± 1.92 |
| Ni2+ | 93.8 ± 3.59 | 91.1 ± 1.94 | 81.2 ± 2.86 |
| Co2+ | 103.7 ± 5.69 | 138.7 ± 4.87 | 93.2 ± 0.99 |
| Cu2+ | 53.3 ± 6.51 | 70.6 ± 3.60 | 64.9 ± 3.16 |
| Zn2+ | 89.1 ± 4.84 | 89.2 ± 2.77 | 109.8 ± 3.22 |
| Mn2+ | 44.5 ± 4.19 | 92.4 ± 4.77 | 120.4 ± 5.40 |
| Mg2+ | 117.7 ± 4.70 | 116.2 ± 3.55 | 70.5 ± 2.15 |
| NH4+ | 103.4 ± 5.83 | 67.8 ± 2.70 | 71.9 ± 1.98 |
| Enzyme | Vmax (μmol/L/min) | Km (g/L) | kcat/Km (L/g·min) | Specific Activity (U/g) | DE Value (%) |
|---|---|---|---|---|---|
| MiXen-CD | (0.33 ± 0.05) a | (1.18 ± 0.02) a | (2.86 ± 0.05) a | (5.1 ± 0.59) a | (0.0092) a |
| MiXen-CBM84 | (6.57 ± 0.98) b | (0.54 ± 0.03) b | (121.7 ± 15.76) b | (82.4 ± 5.18) b | (0.1593) b |
| MiXen-CBMx | (0.98 ± 0.02) c | (0.65 ± 0.02) c | (15.1 ± 0.38) c | (15.7 ± 0.98) c | (0.07) c |
| Enzyme | Xanthan | MiXen-CD | MiXen-CBM84 | MiXen-CBMx |
|---|---|---|---|---|
| HMW | 124.67 | 60.36 | 66.23 | 67.15 |
| LMW | 0 | 20.72 | 43.31 | 28.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, X.; Fu, T.; Wang, X.; Zhao, J.; Yu, Z.; Li, X.; Yang, F. An Insight into the Essential Role of Carbohydrate-Binding Modules in Enzymolysis of Xanthan. Int. J. Mol. Sci. 2023, 24, 5480. https://doi.org/10.3390/ijms24065480
Ni X, Fu T, Wang X, Zhao J, Yu Z, Li X, Yang F. An Insight into the Essential Role of Carbohydrate-Binding Modules in Enzymolysis of Xanthan. International Journal of Molecular Sciences. 2023; 24(6):5480. https://doi.org/10.3390/ijms24065480
Chicago/Turabian StyleNi, Xin, Tong Fu, Xueyan Wang, Jingjing Zhao, Zhimin Yu, Xianzhen Li, and Fan Yang. 2023. "An Insight into the Essential Role of Carbohydrate-Binding Modules in Enzymolysis of Xanthan" International Journal of Molecular Sciences 24, no. 6: 5480. https://doi.org/10.3390/ijms24065480