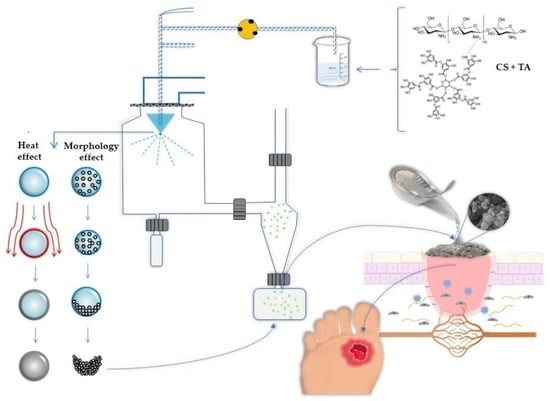
Tannic Acid Tailored-Made Microsystems for Wound Infection
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization of Chitosan Microparticles Loaded Tannic Acid
2.1.1. Product Yield
2.1.2. Particles Characterization: Size Distribution and Morphology
2.1.3. Fourier Transform Infrared Spectroscopy
2.1.4. Differential Scanning Calorimetry
2.1.5. Association Efficiency
2.1.6. In Vitro Release of Tannic Acid from Chitosan Microparticles
2.2. In Vitro Biological Potential of Tannic Acid and Chitosan Microparticles Loaded Tannic Acid
2.2.1. Antioxidant Activity Evaluation
2.2.2. Antimicrobial Potency
2.3. Biocompatibility of Tannic Acid and Chitosan Microparticles Loaded Tannic Acid in Primary Human Dermal Fibroblasts
2.3.1. Particles Morphology with Cells
3. Materials and Methods
3.1. Standards
3.2. Microbial Strains and Inoculum Preparation for Antimicrobial Experiments
3.3. Preparation of Chitosan Microparticles Loaded Tannic Acid
3.4. Physicochemical Characterization of Chitosan Microparticles Loaded Tannic Acid
3.4.1. Product Yield
3.4.2. Particle Characterization: Size Distribution
3.4.3. Fourier-Transform Infrared Analysis
3.4.4. Differential Scanning Calorimetry
3.4.5. Association Efficiency
3.4.6. In Vitro Release of Tannic Acid from Chitosan Microparticles
3.4.7. High Performance Liquid Chromatography Analysis and Tannic Acid Quantification
3.5. In Vitro Biological Potential of Tannic Acid and Chitosan Microparticles Loaded Tannic Acid
3.5.1. Antioxidant Activity Assessment
3.5.2. Antimicrobial Potential
3.6. Cell Culture Experiments
3.6.1. Cells
3.6.2. MTT Viability Assay
3.6.3. BrdU Proliferation Assay
3.6.4. Particles Morphology and Interaction with Cells
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guimarães, I.; Baptista-Silva, S.; Pintado, M.; Oliveira, A.L. Polyphenols: A Promising Avenue in Therapeutic Solutions for Wound Care. Appl. Sci. 2021, 11, 1230. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, A.A.; Pauzi, N.A.S.; Arulselvan, P.; Abas, F.; Fakurazi, S. In Vitro Wound Healing Potential and Identification of Bioactive Compounds from Moringa oleifera Lam. Biomed Res. Int. 2013, 2013, 974580. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S. Wound Healing and Antioxidant Properties: Do They Coexist in Plants? Free. Radic. Antioxid. 2012, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Ustuner, O.; Anlas, C.; Bakirel, T.; Ustun-Alkan, F.; Diren Sigirci, B.; Ak, S.; Akpulat, H.A.; Donmez, C.; Koca-Caliskan, U. In Vitro Evaluation of Antioxidant, Anti-Inflammatory, Antimicrobial and Wound Healing Potential of Thymus Sipyleus Boiss. Subsp. Rosulans (Borbas) Jalas. Molecules 2019, 24, 3353. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Weng, B.; Gilkerson, R.; Materon, L.A.; Lozano, K. Development of Tannic Acid/Chitosan/Pullulan Composite Nanofibers from Aqueous Solution for Potential Applications as Wound Dressing. Carbohydr. Polym. 2015, 115, 16–24. [Google Scholar] [CrossRef]
- Chen, L.; Gnanaraj, C.; Arulselvan, P.; El-Seedi, H.; Teng, H. A Review on Advanced Microencapsulation Technology to Enhance Bioavailability of Phenolic Compounds: Based on Its Activity in the Treatment of Type 2 Diabetes. Trends Food Sci. Technol. 2019, 85, 149–162. [Google Scholar] [CrossRef]
- Alam, P.; Ansari, M.J.; Anwer, M.K.; Raish, M.; Kamal, Y.K.T.; Shakeel, F. Wound Healing Effects of Nanoemulsion Containing Clove Essential Oil. Artif. Cells Nanomed. Biotechnol. 2017, 45, 591–597. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Zheng, Y.; Shen, Y.; Shi, Y.; Li, F.; Su, C.; Zhao, L. Chitosan Nanoparticles Loaded Hydrogels Promote Skin Wound Healing through the Modulation of Reactive Oxygen Species. Artif. Cells Nanomed. Biotechnol. 2018, 46, 138–149. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.B.; Fernandes, J.; Tavaria, F.K.; Pintado, M.; Sarmento, B. The Potential of Chitosan in Drug Delivery Systems; Nova Publishers: New York, NY, USA, 2011; ISBN 9781613244548. [Google Scholar]
- Shariatinia, Z. Pharmaceutical Applications of Chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Dan Mogoşanu, G.; Mihai Grumezescu, A.; Everard Bejenaru, L.; Bejenaru, C. Chapter 8—Natural and Synthetic Polymers for Drug Delivery and Targeting. In Nanobiomaterials in Drug Delivery; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 229–284. ISBN 978-0-323-42866-8. [Google Scholar]
- Casanova, F.; Estevinho, B.N.; Santos, L. Preliminary Studies of Rosmarinic Acid Microencapsulation with Chitosan and Modified Chitosan for Topical Delivery. Powder Technol. 2016, 297, 44–49. [Google Scholar] [CrossRef]
- da Silva, S.B.; Ferreira, D.; Pintado, M.; Sarmento, B. Chitosan-Based Nanoparticles for Rosmarinic Acid Ocular Delivery-In Vitro Tests. Int. J. Biol. Macromol. 2016, 84, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.B.; Amorim, M.; Fonte, P.; Madureira, R.; Ferreira, D.; Pintado, M.; Sarmento, B. Natural Extracts into Chitosan Nanocarriers for Rosmarinic Acid Drug Delivery. Pharm. Biol. 2015, 53, 642–652. [Google Scholar] [CrossRef] [Green Version]
- Cabral, B.R.P.; de Oliveira, P.M.; Gelfuso, G.M.; Quintão, T.D.S.C.; Chaker, J.A.; de Oliveira Karnikowski, M.G.; Gris, E.F. Improving Stability of Antioxidant Compounds from Plinia Cauliflora (Jabuticaba) Fruit Peel Extract by Encapsulation in Chitosan Microparticles. J. Food Eng. 2018, 238, 195–201. [Google Scholar] [CrossRef]
- Lucas, J.; Ralaivao, M.; Estevinho, B.N.; Rocha, F. A New Approach for the Microencapsulation of Curcumin by a Spray Drying Method, in Order to Value Food Products. Powder Technol. 2020, 362, 428–435. [Google Scholar] [CrossRef]
- Santos, D.; Maurício, A.C.; Sencadas, V.; Santos, J.D.; Fernandes, M.H.; Gomes, P.S. Spray Drying: An Overview. In Biomaterials—Physics and Chemistry—New Edition; InTech: London, UK, 2018. [Google Scholar]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with Chitosan by Spray Drying for Industry Applications—A Review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Medina-Torres, L.; García-Cruz, E.E.; Calderas, F.; González Laredo, R.F.; Sánchez-Olivares, G.; Gallegos-Infante, J.A.; Rocha-Guzmán, N.E.; Rodríguez-Ramírez, J. Microencapsulation by Spray Drying of Gallic Acid with Nopal Mucilage (Opuntia Ficus Indica). LWT—Food Sci. Technol. 2013, 50, 642–650. [Google Scholar] [CrossRef]
- Katsarov, P.D.; Pilicheva, B.A.; Manev, H.M.; Lukova, P.K.; Kassarova, M.I. Optimization of Chitosan Microspheres Spray Drying via 32 Full Factorial Design. Folia Med. 2017, 59, 310–317. [Google Scholar] [CrossRef] [Green Version]
- Ziaee, A.; Albadarin, A.B.; Padrela, L.; Femmer, T.; O’Reilly, E.; Walker, G. Spray Drying of Pharmaceuticals and Biopharmaceuticals: Critical Parameters and Experimental Process Optimization Approaches. Eur. J. Pharm. Sci. 2019, 127, 300–318. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds; a Review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosaraju, S.L.; D’ath, L.; Lawrence, A. Preparation and Characterisation of Chitosan Microspheres for Antioxidant Delivery. Carbohydr. Polym. 2006, 64, 163–167. [Google Scholar] [CrossRef]
- Marisa Ribeiro, A.; Estevinho, B.N.; Rocha, F. Microencapsulation of Polyphenols—The Specific Case of the Microencapsulation of Sambucus Nigra L. Extracts—A Review. Trends Food Sci. Technol. 2020, 105, 454–467. [Google Scholar] [CrossRef]
- Kim, H.S.; Sun, X.; Lee, J.-H.; Kim, H.-W.; Fu, X.; Leong, K.W. Advanced Drug Delivery Systems and Artificial Skin Grafts for Skin Wound Healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef] [PubMed]
- Escárega-Galaz, A.A.; López-Cervantes, J.; Sánchez-Machado, D.I.; Brito-Zurita, O.R.; Campas-Baypoli, O.N. Antimicrobial Activity of Chitosan Membranes Against Staphylococcus Aureus of Clinical Origin. In The Rise of Virulence and Antibiotic Resistance in Staphylococcus aureus; InTech: London, UK, 2017. [Google Scholar]
- Jing, Y.; Diao, Y.; Yu, X. Free Radical-Mediated Conjugation of Chitosan with Tannic Acid: Characterization and Antioxidant Capacity. React. Funct. Polym. 2019, 135, 16–22. [Google Scholar] [CrossRef]
- Wahyono, T.; Astuti, D.A.; Komang Gede Wiryawan, I.; Sugoro, I.; Jayanegara, A. Fourier Transform Mid-Infrared (FTIR) Spectroscopy to Identify Tannin Compounds in the Panicle of Sorghum Mutant Lines. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 042045. [Google Scholar] [CrossRef]
- Dey, S.C.; Al-Amin, M.; Rashid, T.U.; Sultan, Z.; Ashaduzzaman; Sarker, M.; Shamsuddin, S.M. Preparation, Characterization and Performance Evaluation of Chitosan as an Adsorbent for Remazol Red. Int. J. Latest Res. Eng. Technol. 2016, 2, 52–62. [Google Scholar]
- Abulateefeh, S.R.; Taha, M.O. Enhanced Drug Encapsulation and Extended Release Profiles of Calcium–Alginate Nanoparticles by Using Tannic Acid as a Bridging Cross-Linking Agent. J. Microencapsul. 2015, 32, 96–105. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Kang, Y.; Li, G. The Interaction between Chitosan and Tannic Acid Calculated Based on the Density Functional Theory. Chem. Phys. 2019, 520, 100–107. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kaczmarek, B.; Lewandowska, K. Modification of Collagen and Chitosan Mixtures by the Addition of Tannic Acid. J. Mol. Liq. 2014, 199, 318–323. [Google Scholar] [CrossRef]
- Mradu, G.; Saumyakanti, S.; Sohini, M.; Arup, M. HPLC Profiles of Standard Phenolic Compounds Present in Medicinal Plants. Int. J. Pharmacogn. Phytochem. Res. 2012, 4, 162–167. [Google Scholar]
- Aelenei, N.; Popa, M.I.; Novac, O.; Lisa, G.; Balaita, L. Tannic Acid Incorporation in Chitosan-Based Microparticles and in Vitro Controlled Release. J. Mater. Sci. Mater. Med. 2009, 20, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.M.; Cochrane, C.A.; Percival, S.L. The Effect of PH on the Extracellular Matrix and Biofilms. Adv. Wound Care 2015, 4, 431–439. [Google Scholar] [CrossRef] [Green Version]
- Nagoba, B.; Suryawanshi, N.M.; Wadher, B.J.; Selkar, S.P. Acidic Environment and Wound Healing: A Review. Wounds-A Compend. Clin. Res. Pract. 2015, 27, 5–11. [Google Scholar]
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; de Matas, M.; Sikstone, V.; Hussain, Z.; Abbasi, M.; Kousar, M. Biopolymer-Based Biomaterials for Accelerated Diabetic Wound Healing: A Critical Review. Int. J. Biol. Macromol. 2019, 139, 975–993. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug Delivery Systems and Materials for Wound Healing Applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef] [PubMed]
- Lončarević, A.; Ivanković, M.; Rogina, A. Lysozyme-Induced Degradation of Chitosan: The Characterisation of Degraded Chitosan Scaffolds. J. Tissue Repair Regen. 2017, 1, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Panico, A.; Paladini, F.; Pollini, M. Development of Regenerative and Flexible Fibroin-based Wound Dressings. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.H.; Zoetendal, E.; Mackie, R.I. Bacterial Mechanisms to Overcome Inhibitory Effects of Dietary Tannins. Microb. Ecol. 2005, 50, 197–205. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and Challenges of Tannins as an Alternative to In-Feed Antibiotics for Farm Animal Production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef]
- Dong, G.; Liu, H.; Yu, X.; Zhang, X.; Lu, H.; Zhou, T.; Cao, J. Antimicrobial and Anti-Biofilm Activity of Tannic Acid against Staphylococcus Aureus. Nat. Prod. Res. 2018, 32, 2225–2228. [Google Scholar] [CrossRef] [PubMed]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Rezazadeh, N.H.; Buazar, F.; Matroodi, S. Synergistic Effects of Combinatorial Chitosan and Polyphenol Biomolecules on Enhanced Antibacterial Activity of Biofunctionalized Silver Nanoparticles. Sci. Rep. 2020, 10, 19615. [Google Scholar] [CrossRef] [PubMed]
- Lamei, E.; Hasanzadeh, M. Fabrication of Chitosan Nanofibrous Scaffolds Based on Tannic Acid and Metal-Organic Frameworks for Hemostatic Wound Dressing Applications. Int. J. Biol. Macromol. 2022, 208, 409–420. [Google Scholar] [CrossRef]
- Su, R.; Li, P.; Zhang, Y.; Lv, Y.; Wen, F.; Su, W. Polydopamine/Tannic Acid/Chitosan/Poloxamer 407/188 Thermosensitive Hydrogel for Antibacterial and Wound Healing. Carbohydr. Polym. 2023, 302, 120349. [Google Scholar] [CrossRef]
- Pattarayan, D.; Sivanantham, A.; Bethunaickan, R.; Palanichamy, R.; Rajasekaran, S. Tannic Acid Modulates Fibroblast Proliferation and Differentiation in Response to Pro-Fibrotic Stimuli. J. Cell. Biochem. 2018, 119, 6732–6742. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Ghaffari-bohlouli, P.; Podstawczyk, D.; Nie, L.; Shavandi, A. Tannic Acid Post-Treatment of Enzymatically Crosslinked Chitosan-Alginate Hydrogels for Biomedical Applications. Carbohydr. Polym. 2022, 295, 119844. [Google Scholar] [CrossRef]
- Ma, D.; Zheng, B.; Du, H.; Han, X.; Zhang, X.; Zhang, J.; Gao, Y.; Sun, S.; Chu, L. The Mechanism Underlying the Protective Effects of Tannic Acid Against Isoproterenol-Induced Myocardial Fibrosis in Mice. Front. Pharmacol. 2020, 11, 716. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Yeh, M.-K.; Cheng, S.-N.; Chiang, C.-H. The Characteristics of Betamethasone-Loaded Chitosan Microparticles by Spray-Drying Method. J. Microencapsul. 2003, 20, 459–472. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Damas, A.M.; Martins, P.; Rocha, F. Microencapsulation of β-Galactosidase with Different Biopolymers by a Spray-Drying Process. Food Res. Int. 2014, 64, 134–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerchiara, T.; Abruzzo, A.; di Cagno, M.; Bigucci, F.; Bauer-Brandl, A.; Parolin, C.; Vitali, B.; Gallucci, M.C.; Luppi, B. Chitosan Based Micro- and Nanoparticles for Colon-Targeted Delivery of Vancomycin Prepared by Alternative Processing Methods. Eur. J. Pharm. Biopharm. 2015, 92, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Rigon, R.T.; Zapata Noreña, C.P. Microencapsulation by Spray-Drying of Bioactive Compounds Extracted from Blackberry (Rubus fruticosus). J. Food Sci. Technol. 2016, 53, 1515–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Fu, X. Cell Behavior on Microparticles with Different Surface Morphology. J. Alloys Compd. 2010, 493, 246–251. [Google Scholar] [CrossRef]







| ABTS (eq. [Trolox] µmol/g) | |
|---|---|
| TA | 917.9 ± 33.0 |
| CS | 160.0 ± 10.2 |
| CMTA | 832.2 ± 80.8 |
| Inhibition Bacterial Growth Zone (cm) | |||||
|---|---|---|---|---|---|
| Staphylococcus aureus(MRSA) | Concentration (mg/mL) | TA | CMTA | CS | Acetic Acid |
| 10 | 1.65 ± 0.10 | 1.10 ± 0.14 | 1.40 ± 0.10 | - | |
| 8 | 1.55 ± 0.10 | 1.10 ± 0.00 | 1.3 ± 0.14 | - | |
| 6 | 1.55 ± 0.10 | 1.05 ± 0.07 | 0.80 ± 0.00 | - | |
| 4 | 1.35 ± 0.10 | - | - | - | |
| 2 | 0.90 ± 0.00 | - | - | - | |
| 1 | - | - | - | - | |
| Staphylococcus aureus(MSSA) | 10 | 1.75 ± 0.07 | 1.15 ± 0.10 | 1.20 ± 0.35 | - |
| 8 | 1.40 ± 0.00 | 1.10 ± 0.00 | 1.1 ± 0.07 | - | |
| 6 | 1.45 ± 0.07 | 0.90 ± 0.00 | 0.8 ± 0.00 | - | |
| 4 | 1.45 ± 0.07 | - | - | - | |
| 2 | 0.90 ± 0.14 | - | - | - | |
| 1 | - | - | - | - | |
| Staphylococcus epidermidis | 10 | 1.45 ± 0.07 | 1.20 ± 0.28 | 1.30 ± 0.42 | - |
| 8 | 1.40 ± 0.14 | 0.90 ± 0.00 | 1.20 ± 0.00 | - | |
| 6 | 1.40 ± 0.00 | 0.50 ± 0.01 | 1.10 ± 0.00 | - | |
| 4 | 1.15 ± 0.10 | - | - | - | |
| 2 | 0.85 ± 0.07 | - | - | - | |
| 1 | - | - | - | - | |
| Escherichia coli | 10 | 1.40 ± 0.00 | 0.80 ± 0.00 | 1.10 ± 0.14 | - |
| 8 | 1.30 ± 0.14 | 0.65 ± 0.02 | 1.00 ± 0.10 | - | |
| 6 | 1.30 ± 0.10 | 0.60 ± 0.14 | 1.00 ± 0.10 | - | |
| 4 | 1.25 ± 0.10 | 0.60 ± 0.00 | 0.90 ± 0.02 | - | |
| 2 | 1.00 ± 0.14 | - | - | - | |
| 1 | - | - | - | - | |
| Candida albicans | 10 | 1.40 ± 0.00 | 1.00 ± 0.00 | 1.30 ± 0.00 | - |
| 8 | 1.30 ± 0.10 | 0.80 ± 0.14 | 1.20 ± 0.14 | - | |
| 6 | 1.30 ± 0.14 | 0.75 ± 0.10 | 1.20 ± 0.10 | - | |
| 4 | 1.25 ± 0.07 | 0.75 ± 0.07 | 1.10 ± 0.00 | - | |
| 2 | 1.00 ± 0.00 | - | - | - | |
| 1 | - | - | - | - | |
| Pseudomonas aeruginosa | |||||
| - | no inhibition zones | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guimarães, I.; Costa, R.; Madureira, S.; Borges, S.; Oliveira, A.L.; Pintado, M.; Baptista-Silva, S. Tannic Acid Tailored-Made Microsystems for Wound Infection. Int. J. Mol. Sci. 2023, 24, 4826. https://doi.org/10.3390/ijms24054826
Guimarães I, Costa R, Madureira S, Borges S, Oliveira AL, Pintado M, Baptista-Silva S. Tannic Acid Tailored-Made Microsystems for Wound Infection. International Journal of Molecular Sciences. 2023; 24(5):4826. https://doi.org/10.3390/ijms24054826
Chicago/Turabian StyleGuimarães, Inês, Raquel Costa, Sara Madureira, Sandra Borges, Ana L. Oliveira, Manuela Pintado, and Sara Baptista-Silva. 2023. "Tannic Acid Tailored-Made Microsystems for Wound Infection" International Journal of Molecular Sciences 24, no. 5: 4826. https://doi.org/10.3390/ijms24054826