Ethosomal Gel for Topical Administration of Dimethyl Fumarate in the Treatment of HSV-1 Infections
Abstract
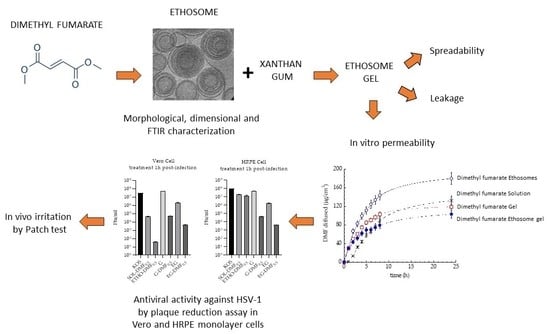
:1. Introduction
2. Results
2.1. Preparation of Ethosomes
2.2. Characterization of Ethosomes
2.2.1. Size Distribution
2.2.2. Morphology
2.2.3. Fourier-Transform Infrared Spectroscopy (FTIR) Studies
Solvent-Removing Experiments
Temperature-Dependent Studies
2.3. DMF Entrapment Capacity (EC)
2.4. Preparation and Characterization of ETHO Gel
2.5. In Vitro Release Test (IVRT)
2.6. In Vitro Permeation Test (IVPT)
2.7. Citotoxicity Evaluation
2.8. In Vitro Antiviral Activity


2.9. Patch Test
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Ethosome Preparation
4.3. Photon Correlation Spectroscopy
4.4. Cryo-Transmission Electron Microscopy
4.5. Structural Characterization of ETHO by FTIR
4.6. Evaluation of DMF EC in Ethosome
4.7. Preparation and Characterization of Ethosomal Gels
4.7.1. Spreadability Studies
4.7.2. Leakage Test
4.8. Franz Cell Diffusion Experiments
4.8.1. In Vitro Release Test (IVRT)
4.8.2. In Vitro Skin Permeation Test (IVPT)
4.9. HPLC Analysis
4.10. Antiviral Activity Study against HSV-1
4.10.1. Cell Culture
4.10.2. Cytotoxicity
4.10.3. Herpes Virus Stock Generation
4.10.4. Titration of Virus by Plaque Assay
4.10.5. Antiviral Activity Assay
4.11. Statistical Analysis
4.12. Patch Test
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Everett, R.D. HSV-1 Biology and Life Cycle. Methods Mol. Biol. 2014, 1144, 1–17. [Google Scholar] [CrossRef]
- Amin, I.; Vajeeha, A.; Younas, S.; Afzal, S.; Shahid, M.; Nawaz, R.; Khan, M.U.; Idrees, M. HSV-1 Infection: Role of Viral Proteins and Cellular Receptors. Crit. Rev. Eukaryot. Gene Expr. 2019, 29, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Arduino, P.G.; Porter, S. Herpes Simplex Virus Type 1 infection: Overview on relevant clinico-pathological features: HSV-1 Literature Review. J. Oral Pathol. Med. 2007, 37, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Y.; Li, X.; Xia, Y.; Wang, E.; Gong, D.; Chen, G.; Yang, L.; Zhang, K.; Zhao, Z.; et al. HSV-1 infection and pathogenesis in the tree shrew eye following corneal inoculation. J. NeuroVirol. 2020, 26, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Knickelbein, J.E.; Hendricks, R.L.; Charukamnoetkanok, P. Management of Herpes Simplex Virus Stromal Keratitis: An Evidence-based Review. Surv. Ophthalmol. 2009, 54, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Tornerup, N.R.; Fomsgaard, A.; Nielsen, N.V. HSV-1–induced acute retinal necrosis syndrome presenting with severe inflammatory orbitopathy, proptosis, and optic nerve involvement. Ophthalmology 2000, 107, 397–401. [Google Scholar] [CrossRef]
- Cruz, G.P.; Fonseca, C.; Oliveira, J.; Da Cunha, J.S. Acute retinal necrosis by herpes simplex virus type 1: An unusual presentation of a primary infection. BMJ Case Rep. 2019, 12, e232566. [Google Scholar] [CrossRef]
- Zhu, H.; Zheng, C. The Race between Host Antiviral Innate Immunity and the Immune Evasion Strategies of Herpes Simplex Virus 1. Microbiol. Mol. Biol. Rev. 2020, 84, e00099-20. [Google Scholar] [CrossRef]
- Arduino, P.G.; Porter, S. Oral and perioral herpes simplex virus type 1 (HSV-1) infection: Review of its management. Oral Dis. 2006, 12, 254–270. [Google Scholar] [CrossRef]
- Heiligenhaus, A.; Li, H.; Schmitz, A.; Wasmuth, S.; Bauer, D. Improvement of herpetic stromal keratitis with fumaric acid derivate is associated with systemic induction of T helper 2 cytokines. Clin. Exp. Immunol. 2005, 142, 180–187. [Google Scholar] [CrossRef]
- Heiligenhaus, A.; Li, H.; Wasmuth, S.; Bauer, D. Influence of dimethylfumarate on experimental HSV-1 necrotizing keratitis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004, 242, 870–877. [Google Scholar] [CrossRef]
- Matteo, P.; Federico, D.; Emanuela, M.; Giulia, R.; Tommaso, B.; Alfredo, G.; Anna, C.; Annamaria, O. New and Old Horizons for an Ancient Drug: Pharmacokinetics, Pharmacodynamics, and Clinical Perspectives of Dimethyl Fumarate. Pharmaceutics 2022, 14, 2732. [Google Scholar] [CrossRef] [PubMed]
- Reszke, R.; Szepietowski, J.C. A safety evaluation of dimethyl fumarate in moderate-to-severe psoriasis. Expert Opin. Drug Saf. 2020, 19, 373–380. [Google Scholar] [CrossRef]
- Mrowietz, U.; Barker, J.; Boehncke, W.-H.; Iversen, L.; Kirby, B.; Naldi, L.; Reich, K.; Tanew, A.; Van De Kerkhof, P.; Warren, R. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: A European expert consensus. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Blair, H.A. Dimethyl Fumarate: A Review in Relapsing-Remitting MS. Drugs 2019, 79, 1965–1976. [Google Scholar] [CrossRef]
- Gill, A.J.; Kolson, D.L. Dimethyl fumarate modulation of immune and antioxidant responses: Application to HIV therapy. Crit. Rev. Immunol. 2013, 33, 307–359. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Li, Y.; Li, Z.; Li, H.; Chen, Y.; Chen, X.; Su, W.; Liang, D. Subconjunctival injections of dimethyl fumarate inhibit lymphangiogenesis and allograft rejection in the rat cornea. Int. Immunopharmacol. 2021, 96, 107580. [Google Scholar] [CrossRef] [PubMed]
- Manai, F.; Govoni, S.; Amadio, M. The Challenge of Dimethyl Fumarate Repurposing in Eye Pathologies. Cells 2022, 11, 4061. [Google Scholar] [CrossRef]
- Li, Y.; Ma, F.; Li, H.; Song, Y.; Zhang, H.; Jiang, Z.; Wu, H. Dimethyl fumarate accelerates wound healing under diabetic conditions. J. Mol. Endocrinol. 2018, 61, 163–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrara, F.; Benedusi, M.; Cervellati, F.; Sguizzato, M.; Montesi, L.; Bondi, A.; Drechsler, M.; Pula, W.; Valacchi, G.; Esposito, E. Dimethyl Fumarate-Loaded Transethosomes: A Formulative Study and Preliminary Ex Vivo and In Vivo Evaluation. Int. J. Mol. Sci. 2022, 23, 8756. [Google Scholar] [CrossRef]
- Natsheh, H.; Vettorato, E.; Touitou, E. Ethosomes for Dermal Administration of Natural Active Molecules. Curr. Pharm. Des. 2019, 25, 2338–2348. [Google Scholar] [CrossRef] [PubMed]
- Touitou, E.; Natsheh, H. Topical Administration of Drugs Incorporated in Carriers Containing Phospholipid Soft Vesicles for the Treatment of Skin Medical Conditions. Pharmaceutics 2021, 13, 2129. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Calderan, L.; Galvan, A.; Cappellozza, E.; Drechsler, M.; Mariani, P.; Pepe, A.; Sguizzato, M.; Vigato, E.; Pozza, E.D.; et al. Ex Vivo Evaluation of Ethosomes and Transethosomes Applied on Human Skin: A Comparative Study. Int. J. Mol. Sci. 2022, 23, 15112. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.-N.; Zhang, Y.-T.; Wang, Q.; Xu, L.; Feng, N. Enhanced in vitro and in vivo skin deposition of apigenin delivered using ethosomes. Int. J. Pharm. 2014, 460, 280–288. [Google Scholar] [CrossRef]
- Jain, S.; Tiwary, A.K.; Sapra, B.; Jain, N.K. Formulation and evaluation of ethosomes for transdermal delivery of lamivudine. AAPS PharmSciTech 2007, 8, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, F.; Benedusi, M.; Sguizzato, M.; Cortesi, R.; Baldisserotto, A.; Buzzi, R.; Valacchi, G.; Esposito, E. Ethosomes and Transethosomes as Cutaneous Delivery Systems for Quercetin: A Preliminary Study on Melanoma Cells. Pharmaceutics 2022, 14, 1038. [Google Scholar] [CrossRef]
- Rehman, W.U.; Asim, M.; Hussain, S.; Khan, S.A.; Khan, S.B. Hydrogel: A Promising Material in Pharmaceutics. Curr. Pharm. Des. 2020, 26, 5892–5908. [Google Scholar] [CrossRef]
- Ballell-Hosa, L.; González-Mira, E.; Santana, H.; Morla-Folch, J.; Moreno-Masip, M.; Martínez-Prieto, Y.; Revuelta, A.; Di Mauro, P.P.; Veciana, J.; Sala, S.; et al. DELOS Nanovesicles-Based Hydrogels: An Advanced Formulation for Topical Use. Pharmaceutics 2022, 14, 199. [Google Scholar] [CrossRef]
- Thakur, K.; Sharma, G.; Singh, B.; Chhibber, S.; Katare, O.P. Current State of Nanomedicines in the Treatment of Topical Infectious Disorders. Recent Pat. Anti-Infect. Drug Discov. 2018, 13, 127–150. [Google Scholar] [CrossRef]
- Qasim, M.; Lim, D.-J.; Park, H.; Na, D. Nanotechnology for Diagnosis and Treatment of Infectious Diseases. J. Nanosci. Nanotechnol. 2014, 14, 7374–7387. [Google Scholar] [CrossRef]
- Reimondez-Troitiño, S.; Csaba, N.; Alonso, M.; de la Fuente, M. Nanotherapies for the treatment of ocular diseases. Eur. J. Pharm. Biopharm. 2015, 95, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Nsengiyumva, E.M.; Alexandridis, P. Xanthan gum in aqueous solutions: Fundamentals and applications. Int. J. Biol. Macromol. 2022, 216, 583–604. [Google Scholar] [CrossRef]
- Mishra, G.P.; Bagui, M.; Tamboli, V.; Mitra, A.K. Recent Applications of Liposomes in Ophthalmic Drug Delivery. J. Drug Deliv. 2011, 2011, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A Review of Poloxamer 407 Pharmaceutical and Pharmacological Characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Liu, X.; Nie, B.; Song, D. FTIR and Raman spectroscopy characterization of functional groups in various rank coals. Fuel 2017, 206, 555–563. [Google Scholar] [CrossRef]
- Cieślik-Boczula, K.; Szwed, J.; Jaszczyszyn, A.; Gasiorowski, K.; Koll, A. Interactions of Dihydrochloride Fluphenazine with DPPC Liposomes: ATR-IR and 31P NMR Studies. J. Phys. Chem. B 2009, 113, 15495–15502. [Google Scholar] [CrossRef]
- Cieślik-Boczula, K.; Koll, A. The effect of 3-pentadecylphenol on DPPC bilayers ATR-IR and 31P NMR studies. Biophys. Chem. 2009, 140, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Ciesik, K.; Koll, A.; Grdadolnik, J. Structural characterization of a phenolic lipid and its derivative using vibrational spectroscopy. Vib. Spectrosc. 2006, 41, 14–20. [Google Scholar] [CrossRef]
- Sydykov, B.; Oldenhof, H.; de Oliveira Barros, L.; Sieme, H.; Wolkers, W.F. Membrane permeabilization of phosphatidylcholine liposomes induced by cryopreservation and vitrification solutions. Biochim. Biophys. Acta (BBA) Biomembr. 2018, 1860, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Golub, P.; Doroshenko, I.; Pogorelov, V.; Sablinskas, V.; Balevicius, V.; Ceponkus, J. Temperature Evolution of Cluster Structures in Ethanol. Dataset Pap. Phys. 2013, 2013, 473294. [Google Scholar] [CrossRef]
- Sicurella, M.; Sguizzato, M.; Cortesi, R.; Huang, N.; Simelière, F.; Montesi, L.; Marconi, P.; Esposito, E. Mangiferin-Loaded Smart Gels for HSV-1 Treatment. Pharmaceutics 2021, 13, 1323. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control Release 2012, 161, 351–362. [Google Scholar] [CrossRef]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® synthetic membrane: Permeability comparison to human cadaver skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef]
- Salamanca, C.H.; Barrera-Ocampo, A.; Lasso, J.C.; Camacho, N.; Yarce, C.J. Franz Diffusion Cell Approach for Pre-Formulation Characterisation of Ketoprofen Semi-Solid Dosage Forms. Pharmaceutics 2018, 10, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sguizzato, M.; Ferrara, F.; Mariani, P.; Pepe, A.; Cortesi, R.; Huang, N.; Simelière, F.; Boldrini, P.; Baldisserotto, A.; Valacchi, G.; et al. “Plurethosome” as Vesicular System for Cutaneous Administration of Mangiferin: Formulative Study and 3D Skin Tissue Evaluation. Pharmaceutics 2021, 13, 1124. [Google Scholar] [CrossRef]
- Sguizzato, M.; Ferrara, F.; Hallan, S.; Baldisserotto, A.; Drechsler, M.; Malatesta, M.; Costanzo, M.; Cortesi, R.; Puglia, C.; Valacchi, G.; et al. Ethosomes and Transethosomes for Mangiferin Transdermal Delivery. Antioxidants 2021, 10, 768. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Cortesi, R.; Drechsler, M.; Fan, J.; Fu, B.M.; Calderan, L.; Mannucci, S.; Boschi, F.; Nastruzzi, C. Nanoformulations for dimethyl fumarate: Physicochemical characterization and in vitro / in vivo behavior. Eur. J. Pharm. Biopharm. 2017, 115, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Drug-Loaded Biocompatible Nanocarriers Embedded in Poloxamer 407 Hydrogels as Therapeutic Formulations. Medicines 2018, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Cristiano, M.; Mancuso, A.; Giuliano, E.; Cosco, D.; Paolino, D.; Fresta, M. EtoGel for Intra-Articular Drug Delivery: A New Challenge for Joint Diseases Treatment. J. Funct. Biomater. 2021, 12, 34. [Google Scholar] [CrossRef]
- Hallan, S.; Sguizzato, M.; Mariani, P.; Cortesi, R.; Huang, N.; Simelière, F.; Marchetti, N.; Drechsler, M.; Ruzgas, T.; Esposito, E. Design and Characterization of Ethosomes for Transdermal Delivery of Caffeic Acid. Pharmaceutics 2020, 12, 740. [Google Scholar] [CrossRef]
- Esposito, E.; Sguizzato, M.; Bories, C.; Nastruzzi, C.; Cortesi, R. Production and Characterization of a Clotrimazole Liposphere Gel for Candidiasis Treatment. Polymers 2018, 10, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, E.; Ravani, L.; Mariani, P.; Contado, C.; Drechsler, M.; Puglia, C.; Cortesi, R. Curcumin containing monoolein aqueous dispersions: A preformulative study. Mater. Sci. Eng. C 2013, 33, 4923–4934. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jain, S.K. In vitro release kinetics model fitting of liposomes: An insight. Chem. Phys. Lipids 2016, 201, 28–40. [Google Scholar] [CrossRef]
- Sguizzato, M.; Mariani, P.; Spinozzi, F.; Benedusi, M.; Cervellati, F.; Cortesi, R.; Drechsler, M.; Prieux, R.; Valacchi, G.; Esposito, E. Ethosomes for Coenzyme Q10 Cutaneous Administration: From Design to 3D Skin Tissue Evaluation. Antioxidants 2020, 9, 485. [Google Scholar] [CrossRef]
- Shen, Y.; Tu, J. Preparation and ocular pharmacokinetics of ganciclovir liposomes. AAPS J. 2007, 9, E371–E377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horwitz, E.; Pisanty, S.; Czerninski, R.; Helser, M.; Eliav, E.; Touitou, E. A clinical evaluation of a novel liposomal carrier for acyclovir in the topical treatment of recurrent herpes labialis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1999, 87, 700–705. [Google Scholar] [CrossRef]
- Mallick, A.; Sahu, R.; Nandi, G.; Dua, T.K.; Shaw, T.K.; Dhar, A.; Kanu, A.; Paul, P. Development of Liposomal Formulation for Controlled Delivery of Valacyclovir: An In Vitro Study. J. Pharm. Innov. 2023. [Google Scholar] [CrossRef]
- Cortesi, R.; Argnani, R.; Esposito, E.; Dalpiaz, A.; Scatturin, A.; Bortolotti, F.; Lufino, M.; Guerrini, R.; Cavicchioni, G.; Incorvaia, C.; et al. Cationic liposomes as potential carriers for ocular administration of peptides with anti-herpetic activity. Int. J. Pharm. 2006, 317, 90–100. [Google Scholar] [CrossRef]
- Ahmed, T.; Alzahrani, M.; Sirwi, A.; Alhakamy, N. Study the Antifungal and Ocular Permeation of Ketoconazole from Ophthalmic Formulations Containing Trans-Ethosomes Nanoparticles. Pharmaceutics 2021, 13, 151. [Google Scholar] [CrossRef]
- Javaid, A.; Zahra, D.; Asim, A.; Javaid, N.; Ashfaq, U.A. Recent Updates on the Role of Nanoparticles in the Treatment of Viral Diseases. Crit. Rev. Ther. Drug Carr. Syst. 2021, 38, 75–102. [Google Scholar] [CrossRef]
- Pecora, R. Dynamic Light Scattering Measurement of Nanometer Particles in Liquids. J. Nanoparticle Res. 2000, 2, 123–131. [Google Scholar] [CrossRef]
- Miranda, M.; Pais, A.; Cardoso, C.; Vitorino, C. aQbD as a platform for IVRT method development—A regulatory oriented approach. Int. J. Pharm. 2019, 572, 118695. [Google Scholar] [CrossRef] [PubMed]
- EMA Quality and Equivalence of Topical Products—Scientific Guideline. Available online: https://www.ema.europa.eu/en/quality-equivalence-topical-products-scientific-guideline (accessed on 24 January 2023).
- Le Guyader, G.; Do, B.; Vieillard, V.; Andrieux, K.; Paul, M. Comparison of the In Vitro and Ex Vivo Permeation of Existing Topical Formulations Used in the Treatment of Facial Angiofibroma and Characterization of the Variations Observed. Pharmaceutics 2020, 12, 1060. [Google Scholar] [CrossRef] [PubMed]
- Sutter, S.O.; Marconi, P.; Meier, A.F. Herpes Simplex Virus Growth, Preparation, and Assay. Methods Mol. Biol. 2019, 2060, 57–72. [Google Scholar] [CrossRef]








| Formulation | PC 1 % w/w | Ethanol % w/w | DMF 2 % w/w | Water % w/w |
|---|---|---|---|---|
| ETHO | 0.90 | 29.10 | - | 70.00 |
| ETHO-DMF0.5 | 0.90 | 29.05 | 0.05 | 70.00 |
| ETHO-DMF1.0 | 0.90 | 29.00 | 0.10 | 70.00 |
| Formulation | Z Average (nm) | Typical Intensity Distribution (nm) | Dispersity Index | SIR * |
|---|---|---|---|---|
| ETHO | 212.25 ± 15.13 | 237.1 (100%) | 0.15 ± 0.07 | 11.9 ± 0.9 |
| ETHO-DMF0.5 | 223.22 ± 13.60 | 234.1 (100%) | 0.12 ± 0.05 | 8.88 ± 0.5 |
| ETHO-DMF1.0 | 231.00 ± 10.81 | 256.5 (94.2%) 4158 (5.8%) | 0.22 ± 0.01 | 16.01 ± 1.2 |
| Formulation | PC 1 % w/w | Ethanol % w/w | Water % w/w | Thickener % w/w | Spreadability 4 (g·cm/s) | Leakage 5 (cm) | |
|---|---|---|---|---|---|---|---|
| x-gum 2 | p-407 3 | ||||||
| ETHO | 0.90 | 29.10 | 70.00 | - | - | 27.50 ± 0.25 | 5.00 ± 0.04 |
| ETHO x-gum0.5 | 0.90 | 29.10 | 69.50 | 0.5 | - | 16.75 ± 3.40 | 1.67 ± 0.40 |
| ETHO x-gum1.0 | 0.90 | 29.10 | 69.00 | 1.0 | - | 12.16 ± 3.00 | 1.72 ± 0.26 |
| ETHO p-40715 | 0.90 | 29.10 | 55.00 | - | 15.0 | 27.50 ± 5.55 | 5.00 ± 0.00 |
| ETHO p-40720 | 0.90 | 29.10 | 50.00 | - | 20.0 | 12.50 ± 3.15 | 1.85 ± 0.61 |
| Formulation | PC 1 % w/w | Ethanol % w/w | Water % w/w | DMF 2 % w/w | x-gum % w/w |
|---|---|---|---|---|---|
| EG-DMF0.5 | 0.90 | 29.05 | 69.50 | 0.05 | 0.50 |
| G-DMF0.5 | - | - | 99.45 | 0.05 | 0.50 |
| EG | 0.90 | 29.10 | 69.50 | - | 0.50 |
| G | - | - | 99.50 | - | 0.50 |
| IVRT Parameters | ETHO-DMF0.5 | SOL-DMF0.5 | EG-DMF0.5 | G-DMF0.5 |
|---|---|---|---|---|
| RDMF 1 ± s.d. (μg/cm2/h) | 64.60 ± 5.40 | 100.66 ± 18.12 | 35.44 ± 3.15 | 68.85± 7.35 |
| Tlag 2 ± s.d. (h) | 0.18 ± 0.01 | 0.15 ± 0.01 | 0.00 ± 0.01 | 0.00 ± 0.01 |
| ADMF 3 ± s.d. (μg/cm2) | 180.00 ± 18.21 | 269.33 ± 42.02 | 98.00 ± 2.02 | 200 ± 20.2 |
| Formulation | Zero Order Plot (R2) | First Order Plot (R2) | Higuchi Plot (R2) | Peppas Plot (n) |
|---|---|---|---|---|
| ETHO-DMF0.5 | 0.9836 | 0.9827 | 0.9841 | 0.6408 |
| EG-DMF0.5 | 0.9578 | 0.9096 | 0.9944 | 0.5311 |
| G-DMF0.5 | 0.9638 | 0.9481 | 0.9974 | 0.5027 |
| IVPT Parameters | ETHO-DMF0.5 | SOL-DMF0.5 | EG-DMF0.5 | G-DMF0.5 |
|---|---|---|---|---|
| Jss 1 (mg cm−2 h−1) | 15.16 ± 1.52 | 14.71 ± 0.40 | 9.77 ± 1.81 | 13.56 ± 2.84 |
| Tlag 2 ± s.d. (h) | 0.00 ± 0.01 | 0.55 ± 0.02 | 0.00 ± 0.01 | 0.00 ± 0.01 |
| Kp 3 (cm h−1 10−3) | 30.32 ± 3.04 | 29.42 ± 0.8 | 19.54 ± 3.62 | 27.12 ± 5.68 |
| ADMF 4 (μg cm−2) | 178.10 ± 10.52 | 132.6 ± 9.2 | 103.33 ± 4.15 | 118.80 ± 6.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sicurella, M.; Pula, W.; Musiał, K.; Cieślik-Boczula, K.; Sguizzato, M.; Bondi, A.; Drechsler, M.; Montesi, L.; Esposito, E.; Marconi, P. Ethosomal Gel for Topical Administration of Dimethyl Fumarate in the Treatment of HSV-1 Infections. Int. J. Mol. Sci. 2023, 24, 4133. https://doi.org/10.3390/ijms24044133
Sicurella M, Pula W, Musiał K, Cieślik-Boczula K, Sguizzato M, Bondi A, Drechsler M, Montesi L, Esposito E, Marconi P. Ethosomal Gel for Topical Administration of Dimethyl Fumarate in the Treatment of HSV-1 Infections. International Journal of Molecular Sciences. 2023; 24(4):4133. https://doi.org/10.3390/ijms24044133
Chicago/Turabian StyleSicurella, Mariaconcetta, Walter Pula, Karolina Musiał, Katarzyna Cieślik-Boczula, Maddalena Sguizzato, Agnese Bondi, Markus Drechsler, Leda Montesi, Elisabetta Esposito, and Peggy Marconi. 2023. "Ethosomal Gel for Topical Administration of Dimethyl Fumarate in the Treatment of HSV-1 Infections" International Journal of Molecular Sciences 24, no. 4: 4133. https://doi.org/10.3390/ijms24044133