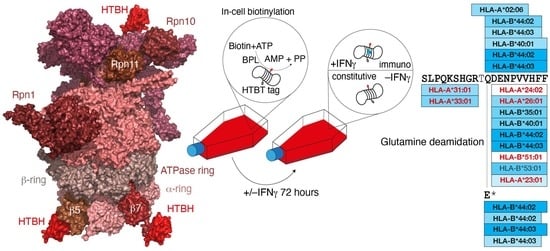
Myelin Basic Protein Fragmentation by Engineered Human Proteasomes with Different Catalytic Phenotypes Revealed Direct Peptide Ligands of MS-Associated and Protective HLA Class I Molecules
Abstract
:1. Introduction
2. Results
2.1. Purification and Characterization of the HTBH-Engineered Proteasomes
2.2. Fragmentation of Myelin Basic Protein by Proteasomes with Different Catalytic Phenotypes
3. Discussion
4. Materials and Methods
4.1. Mammalian Cells Constitutively Expressing HTBH-Tagged Proteasomes
4.2. Purification of the HTBH-Tagged Proteasomes
4.3. Analysis of the Proteasome Activity
4.4. In-Gel Fluorescence with Me4BodipyFL-Ahx3Leu3VS Fluorescent Proteasome Probe
4.5. Native PAGE with LLVY-AMC
4.6. Negative Staining Electron Microscopy
4.7. MBP Hydrolisis by Proteasomes
4.8. Mass Spectrometry
4.9. Bioinformatics
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kudriaeva, A.A.; Belogurov, A.A. Proteasome: A Nanomachinery of Creative Destruction. Biochem. (Mosc.) 2019, 84, 159–192. [Google Scholar] [CrossRef] [PubMed]
- Kudriaeva, A.A.; Sokolov, A.V.; Belogurov, A.A. Stochastics of Degradation: The Autophagic-Lysosomal System of the Cell. Acta Nat. 2020, 12, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Vigneron, N.; Abi Habib, J.; van den Eynde, B.J. Learning from the Proteasome: How To Fine-Tune Cancer Immunotherapy. Trends Cancer 2017, 3, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Luo, H. Interplay between the Virus and the Ubiquitin-Proteasome System: Molecular Mechanism of Viral Pathogenesis. Curr. Opin. Virol. 2016, 17, 1–10. [Google Scholar] [CrossRef]
- Leone, P.; Racanelli, V. Editorial: Targeting Antigen Processing and Presentation in Autoimmune and Autoinflammatory Disorders. Front. Immunol. 2022, 13, 1055152. [Google Scholar] [CrossRef]
- Wagner, C.A.; Roqué, P.J.; Mileur, T.R.; Liggitt, D.; Goverman, J.M. Myelin-Specific CD8+ T Cells Exacerbate Brain Inflammation in CNS Autoimmunity. J. Clin. Investig. 2020, 130, 203–213. [Google Scholar] [CrossRef]
- Mockus, T.E.; Munie, A.; Atkinson, J.R.; Segal, B.M. Encephalitogenic and Regulatory CD8 T Cells in Multiple Sclerosis and Its Animal Models. J. Immunol. 2021, 206, 3–10. [Google Scholar] [CrossRef]
- Steinman, L. Multiple sclerosis: A coordinated immunological attack against myelin in the central nervous system. Cell 1996, 85, 299–302. [Google Scholar] [CrossRef] [Green Version]
- Medveczky, P.; Antal, J.; Patthy, A.; Kékesi, K.; Juhász, G.; Szilágyi, L.; Gráf, L. Myelin basic protein, an autoantigen in multiple sclerosis, is selectively processed by human trypsin 4. FEBS Lett. 2006, 580, 545–552. [Google Scholar] [CrossRef] [Green Version]
- Correale, J.; Gaitán, M.I.; Ysrraelit, M.C.; Fiol, M.P. Progressive multiple sclerosis: From pathogenic mechanisms to treatment. Brain 2017, 140, 527–546. [Google Scholar] [CrossRef]
- Fierabracci, A. Proteasome inhibitors: A new perspective for treating autoimmune diseases. Curr. Drug Targets 2012, 13, 1665–1675. [Google Scholar] [CrossRef]
- Belogurov, A.; Kudriaeva, A.; Kuzina, E.; Smirnov, I.; Bobik, T.; Ponomarenko, N.; Kravtsova-Ivantsiv, Y.; Ciechanover, A.; Gabibov, A. Multiple Sclerosis Autoantigen Myelin Basic Protein Escapes Control by Ubiquitination during Proteasomal Degradation. J. Biol. Chem. 2014, 289, 17758–17766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuzina, E.S.; Chernolovskaya, E.L.; Kudriaeva, A.A.; Zenkova, M.A.; Knorre, V.D.; Surina, E.A.; Ponomarenko, N.A.; Bobik, T.V.; Smirnov, I.V.; Bacheva, A.V.; et al. Immunoproteasome Enhances Intracellular Proteolysis of Myelin Basic Protein. Dokl Biochem. Biophys. 2013, 453, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Kudriaeva, A.; Kuzina, E.S.; Zubenko, O.; Smirnov, I.V.; Belogurov, A.A. Charge-Mediated Proteasome Targeting. FASEB J. 2019, 33, 6852–6866. [Google Scholar] [CrossRef]
- Kuzina, E.S.; Kudriaeva, A.A.; Glagoleva, I.S.; Knorre, V.D.; Gabibov, A.G.; Belogurov, A.A. Deimination of the Myelin Basic Protein Decelerates Its Proteasome-Mediated Metabolism. Dokl Biochem. Biophys. 2016, 469, 277–280. [Google Scholar] [CrossRef]
- Kuzina, E.; Kudriaeva, A.; Smirnov, I.; Dubina, M.; Gabibov, A.; Belogurov, A. Glatiramer Acetate and Nanny Proteins Restrict Access of the Multiple Sclerosis Autoantigen Myelin Basic Protein to the 26S Proteasome. Biomed. Res. Int. 2014, 2014, 926394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belogurov, A.; Kuzina, E.; Kudriaeva, A.; Kononikhin, A.; Kovalchuk, S.; Surina, Y.; Smirnov, I.; Lomakin, Y.; Bacheva, A.; Stepanov, A.; et al. Ubiquitin-Independent Proteosomal Degradation of Myelin Basic Protein Contributes to Development of Neurodegenerative Autoimmunity. FASEB J. 2015, 29, 1901–1913. [Google Scholar] [CrossRef]
- Kulichkova, V.A.; Artamonova, T.O.; Zaykova, J.J.; Ermolaeva, J.B.; Khodorkovskii, M.A.; Barlev, N.A.; Tomilin, A.N.; Tsimokha, A.S. Simultaneous EGFP and Tag Labeling of the Β7 Subunit for Live Imaging and Affinity Purification of Functional Human Proteasomes. Mol. Biotechnol. 2015, 57, 36–44. [Google Scholar] [CrossRef]
- Wang, X.; Chen, C.F.; Baker, P.R.; Chen, P.L.; Kaiser, P.; Huang, L. Mass Spectrometric Characterization of the Affinity-Purified Human 26S Proteasome Complex. Biochemistry 2007, 46, 3553–3565. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, S.; Wu, Z.; Li, X.; Wang, W.L.; Zhu, Y.; Stoilova-McPhie, S.; Lu, Y.; Finley, D.; Mao, Y. Cryo-EM Structures and Dynamics of Substrate-Engaged Human 26S Proteasome. Nature 2019, 565, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Ivics, Z.; Hackett, P.B.; Plasterk, R.H.; Izsvák, Z. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell 1997, 91, 501–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkers, C.R.; van Leeuwen, F.W.B.; Groothuis, T.A.; Peperzak, V.; van Tilburg, E.W.; Borst, J.; Neefjes, J.J.; Ovaa, H. Profiling Proteasome Activity in Tissue with Fluorescent Probes. Mol. Pharm. 2007, 4, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Fabre, B.; Lambour, T.; Garrigues, L.; Ducoux-Petit, M.; Amalric, F.; Monsarrat, B.; Burlet-Schiltz, O.; Bousquet-Dubouch, M.P. Label-Free Quantitative Proteomics Reveals the Dynamics of Proteasome Complexes Composition and Stoichiometry in a Wide Range of Human Cell Lines. J. Proteome Res. 2014, 13, 3027–3037. [Google Scholar] [CrossRef] [PubMed]
- Reinheckel, T.; Sitte, N.; Ullrich, O.; Kuckelkorn, U.; Davies, K.J.; Grune, T. Comparative resistance of the 20S and 26S proteasome to oxidative stress. Biochem. J. 1998, 335, 637–642. [Google Scholar] [CrossRef]
- Al-Nashmi, M.; Taha, S.; Salem, A.H.; Alsharoqi, I.; Bakhiet, M. Distinct HLA Class I and II Genotypes and Haplotypes Are Associated with Multiple Sclerosis in Bahrain. Biomed. Rep. 2018, 9, 531–539. [Google Scholar] [CrossRef] [Green Version]
- Galehdari, H.; Mohaghegh, M.; Majdinasab, N.; Khatami, S.R.; Hosseini Behbahani, M. Analysis of HLA-A*03 in Multiple Sclerosis Patients in Khuzestan Province, Iran. Gene Cell Tissue 2018, 5, e68293. [Google Scholar] [CrossRef] [Green Version]
- McMahon, R.M.; Friis, L.; Siebold, C.; Friese, M.A.; Fugger, L.; Jones, E.Y. Structure of HLA-A*0301 in Complex with a Peptide of Proteolipid Protein: Insights into the Role of HLA—A Alleles in Susceptibility to Multiple Sclerosis. Acta Crystallogr D Biol. Crystallogr. 2011, 67, 447–454. [Google Scholar] [CrossRef]
- Rajaei, S.J.; Shakhsi-Niaei, M.; Etemadifar, M. Association of HLA-A*03/A*31 and HLA-A*24/A*31 Haplotypes with Multiple Sclerosis. J. Cell Mol. Res. 2022, 13, 121–128. [Google Scholar] [CrossRef]
- Jilek, S.; Schluep, M.; Harari, A.; Canales, M.; Lysandropoulos, A.; Zekeridou, A.; Pantaleo, G.; du Pasquier, R.A. HLA-B7–Restricted EBV-Specific CD8 + T Cells Are Dysregulated in Multiple Sclerosis. J. Immunol. 2012, 188, 4671–4680. [Google Scholar] [CrossRef] [Green Version]
- Lomakin, Y.; Arapidi, G.P.; Chernov, A.; Ziganshin, R.; Tcyganov, E.; Lyadova, I.; Butenko, I.O.; Osetrova, M.; Ponomarenko, N.; Telegin, G.; et al. Exposure to the Epstein-Barr Viral Antigen Latent Membrane Protein 1 Induces Myelin-Reactive Antibodies In Vivo. Front. Immunol. 2017, 8, 777. [Google Scholar] [CrossRef]
- Gabibov, A.G.; Belogurov, A.A.; Lomakin, Y.A.; Zakharova, M.Y.; Avakyan, M.E.; Dubrovskaya, V.V.; Smirnov, I.V.; Ivanov, A.S.; Molnar, A.A.; Gurtsevitch, V.E.; et al. Combinatorial Antibody Library from Multiple Sclerosis Patients Reveals Antibodies That Cross-react with Myelin Basic Protein and EBV Antigen. FASEB J. 2011, 25, 4211–4221. [Google Scholar] [CrossRef] [PubMed]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal Analysis Reveals High Prevalence of Epstein-Barr Virus Associated with Multiple Sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Ota, K.; Matsui, M.; Milford, E.L.; Mackin, G.A.; Weiner, H.L.; Hafler, D.A. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature 1990, 346, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Healy, B.C.; Liguori, M.; Tran, D.; Chitnis, T.; Glanz, B.; Wolfish, C.; Gauthier, S.; Buckle, D.G.; Houtchens, M.; Stazzone, L.; et al. HLA B*44 Protective Effects in MS Susceptibility and MRI Outcome Measures. Neurology 2010, 75, 634–640. [Google Scholar] [CrossRef]
- La Mantia, L.; Illeni, M.T.; Milanese, C.; Salmaggi, A.; Eoli, M.; Pellegris, G.; Nespolo, A. HLA Antigens in Italian Multiple Sclerosis Patients. Ital. J. Neurol. Sci. 1991, 12, 81–86. [Google Scholar] [CrossRef]
- Malinowski, S.M.; Pulido, J.S.; Goeken, N.E.; Brown, C.K.; Folk, J.C. The Association of HLA-B8, B51, DR2, and Multiple Sclerosis in Pars Planitis. Ophthalmology 1993, 100, 1199–1205. [Google Scholar] [CrossRef]
- Prieto, J.F.; Dios, E.; Gutierrez, J.M.; Mayo, A.; Calonge, M.; Herreras, J.M. Pars planitis: Epidemiology, treatment, and association with multiple sclerosis. Ocul. Immunol. Inflamm. 2001, 9, 93–102. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, S.I.; Pernis, B. Role of CD8+ T cells in murine experimental allergic encephalomyelitis. Science 1992, 256, 1213–1215. [Google Scholar] [CrossRef]
- Saligrama, N.; Zhao, F.; Sikora, M.J.; Serratelli, W.S.; Fernandes, R.A.; Louis, D.M.; Yao, W.; Ji, X.; Idoyaga, J.; Mahajan, V.B.; et al. Opposing T Cell Responses in Experimental Autoimmune Encephalomyelitis. Nature 2019, 572, 481–487. [Google Scholar] [CrossRef]
- Limanaqi, F.; Biagioni, F.; Gaglione, A.; Busceti, C.L.; Fornai, F. A Sentinel in the Crosstalk Between the Nervous and Immune System: The (Immuno)-Proteasome. Front. Immunol. 2019, 10, 628. [Google Scholar] [CrossRef]
- Tundo, G.R.; Sbardella, D.; Oddone, F.; Kudriaeva, A.A.; Lacal, P.M.; Belogurov, A.A.; Graziani, G.; Marini, S. At the Cutting Edge against Cancer: A Perspective on Immunoproteasome and Immune Checkpoints Modulation as a Potential Therapeutic Intervention. Cancers 2021, 13, 4852. [Google Scholar] [CrossRef] [PubMed]
- Elsasser, S.; Schmidt, M.; Finley, D. Characterization of the proteasome using native gel electrophoresis. Methods Enzymol. 2005, 398, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, S.I.; Jensen, O.N.; Rogowska-Wrzesinska, A. FlashPack: Fast and Simple Preparation of Ultrahigh-performance Capillary Columns for LC-MS. Mol. Cell. Proteom. 2019, 18, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynisson, B.; Alvarez, B.; Paul, S.; Peters, B.; Nielsen, M. NetMHCpan-4.1 and NetMHCIIpan-4.0: Improved Predictions of MHC Antigen Presentation by Concurrent Motif Deconvolution and Integration of MS MHC Eluted Ligand Data. Nucleic Acids Res. 2021, 48, W449–W454. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saratov, G.A.; Vladimirov, V.I.; Novoselov, A.L.; Ziganshin, R.H.; Chen, G.; Baymukhametov, T.N.; Konevega, A.L.; Belogurov, A.A., Jr.; Kudriaeva, A.A. Myelin Basic Protein Fragmentation by Engineered Human Proteasomes with Different Catalytic Phenotypes Revealed Direct Peptide Ligands of MS-Associated and Protective HLA Class I Molecules. Int. J. Mol. Sci. 2023, 24, 2091. https://doi.org/10.3390/ijms24032091
Saratov GA, Vladimirov VI, Novoselov AL, Ziganshin RH, Chen G, Baymukhametov TN, Konevega AL, Belogurov AA Jr., Kudriaeva AA. Myelin Basic Protein Fragmentation by Engineered Human Proteasomes with Different Catalytic Phenotypes Revealed Direct Peptide Ligands of MS-Associated and Protective HLA Class I Molecules. International Journal of Molecular Sciences. 2023; 24(3):2091. https://doi.org/10.3390/ijms24032091
Chicago/Turabian StyleSaratov, George A., Vasiliy I. Vladimirov, Alexey L. Novoselov, Rustam H. Ziganshin, Guo Chen, Timur N. Baymukhametov, Andrey L. Konevega, Alexey A. Belogurov, Jr., and Anna A. Kudriaeva. 2023. "Myelin Basic Protein Fragmentation by Engineered Human Proteasomes with Different Catalytic Phenotypes Revealed Direct Peptide Ligands of MS-Associated and Protective HLA Class I Molecules" International Journal of Molecular Sciences 24, no. 3: 2091. https://doi.org/10.3390/ijms24032091