Hemodialysis-Associated Immune Dysregulation in SARS-CoV-2-Infected End-Stage Renal Disease Patients
Abstract
:1. Introduction
2. Results
2.1. Long-Term HD Patient Characteristics
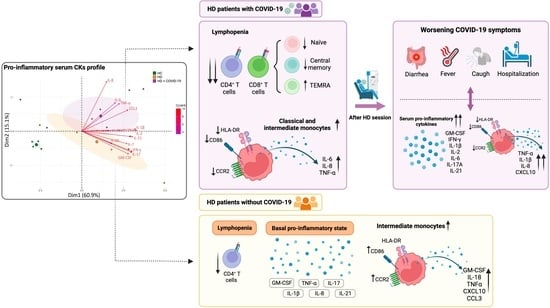
2.2. Long-Term HD Patients Showed a Pro-Inflammatory Serum Cytokine Profile Which Increased with COVID-19
2.3. Effect of COVID-19 on Lymphocyte and Monocyte Phenotype and Function in Chronic HD Patients
2.4. Increased Inflammatory Status and Capacity of Monocytes to Secrete Cytokines in Patients Who Worsen Their COVID-19 Symptoms after HD Sessions
3. Discussion
4. Materials and Methods
4.1. Patients and Controls
4.2. Analysis of Lymphocyte and Monocyte Subsets by Flow Cytometry
4.3. Monocyte In Vitro Cultures
4.4. Multiplex Detection of Cytokines
4.5. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jager, K.J.; Kramer, A.; Chesnaye, N.C.; Couchoud, C.; Sanchez-Alvarez, J.E.; Garneata, L.; Collart, F.; Hemmelder, M.H.; Ambuhl, P.; Kerschbaum, J.; et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020, 98, 1540–1548. [Google Scholar] [CrossRef]
- Sim, J.J.; Huang, C.W.; Selevan, D.C.; Chung, J.; Rutkowski, M.P.; Zhou, H. COVID-19 and Survival in Maintenance Dialysis. Kidney Med. 2021, 3, 132–135. [Google Scholar] [CrossRef]
- Corbett, R.W.; Blakey, S.; Nitsch, D.; Loucaidou, M.; McLean, A.; Duncan, N.; Ashby, D.R.; West London, R.; Transplant, C. Epidemiology of COVID-19 in an Urban Dialysis Center. J. Am. Soc. Nephrol. 2020, 31, 1815–1823. [Google Scholar] [CrossRef]
- Ng, J.H.; Hirsch, J.S.; Wanchoo, R.; Sachdeva, M.; Sakhiya, V.; Hong, S.; Jhaveri, K.D.; Fishbane, S.; Northwell, C.-R.C.; the Northwell Nephrology, C.-R.C. Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int. 2020, 98, 1530–1539. [Google Scholar] [CrossRef]
- Valeri, A.M.; Robbins-Juarez, S.Y.; Stevens, J.S.; Ahn, W.; Rao, M.K.; Radhakrishnan, J.; Gharavi, A.G.; Mohan, S.; Husain, S.A. Presentation and Outcomes of Patients with ESKD and COVID-19. J. Am. Soc. Nephrol. 2020, 31, 1409–1415. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Sánchez-Álvarez, J.E.; Fontán, M.P.; Martín, C.J.; Pelícano, M.B.; Reina, C.J.C.; Prieto, Á.M.S.; Melilli, E.; Barrios, M.C.; Heras, M.M.; Pino, M.D.d.P.y. Status of SARS-CoV-2 infection in patients on renal replacement therapy. Report of the COVID-19 Registry of the Spanish Society of Nephrology (SEN). Nefrología 2020, 40, 272–278. [Google Scholar] [CrossRef]
- Collier, S.; Davenport, A. Reducing the risk of infection in end-stage kidney failure patients treated by dialysis. Nephrol. Dial. Transplant. 2014, 29, 2158–2161. [Google Scholar] [CrossRef] [Green Version]
- Campo, S.; Lacquaniti, A.; Trombetta, D.; Smeriglio, A.; Monardo, P. Immune System Dysfunction and Inflammation in Hemodialysis Patients: Two Sides of the Same Coin. J. Clin. Med. 2022, 11, 3759. [Google Scholar] [CrossRef]
- Syed-Ahmed, M.; Narayanan, M. Immune Dysfunction and Risk of Infection in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2019, 26, 8–15. [Google Scholar] [CrossRef]
- Xiang, F.; Cao, X.; Chen, X.; Zhang, Z.; Ding, X.; Zou, J.; Shen, B. Decreased Peripheral Naive T Cell Number and Its Role in Predicting Cardiovascular and Infection Events in Hemodialysis Patients. Front. Immunol. 2021, 12, 644627. [Google Scholar] [CrossRef]
- Molina, M.; Allende, L.M.; Ramos, L.E.; Gutierrez, E.; Pleguezuelo, D.E.; Hernandez, E.R.; Rios, F.; Fernandez, C.; Praga, M.; Morales, E. CD19(+) B-Cells, a New Biomarker of Mortality in Hemodialysis Patients. Front. Immunol. 2018, 9, 1221. [Google Scholar] [CrossRef] [Green Version]
- Meier, P.; Dayer, E.; Blanc, E.; Wauters, J.P. Early T cell activation correlates with expression of apoptosis markers in patients with end-stage renal disease. J. Am. Soc. Nephrol. 2002, 13, 204–212. [Google Scholar] [CrossRef]
- Betjes, M.G.; Langerak, A.W.; van der Spek, A.; de Wit, E.A.; Litjens, N.H. Premature aging of circulating T cells in patients with end-stage renal disease. Kidney Int. 2011, 80, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Fresnedo, G.; Ramos, M.A.; Gonzalez-Pardo, M.C.; de Francisco, A.L.; Lopez-Hoyos, M.; Arias, M. B lymphopenia in uremia is related to an accelerated in vitro apoptosis and dysregulation of Bcl-2. Nephrol. Dial. Transplant. 2000, 15, 502–510. [Google Scholar] [CrossRef] [Green Version]
- Westphalen, H.; Saadati, S.; Eduok, U.; Abdelrasoul, A.; Shoker, A.; Choi, P.; Doan, H.; Ein-Mozaffari, F. Case studies of clinical hemodialysis membranes: Influences of membrane morphology and biocompatibility on uremic blood-membrane interactions and inflammatory biomarkers. Sci. Rep. 2020, 10, 14808. [Google Scholar] [CrossRef]
- Borges Bonan, N.; Schepers, E.; Pecoits-Filho, R.; Dhondt, A.; Pletinck, A.; De Somer, F.; Vanholder, R.; Van Biesen, W.; Moreno-Amaral, A.; Glorieux, G. Contribution of the uremic milieu to an increased pro-inflammatory monocytic phenotype in chronic kidney disease. Sci. Rep. 2019, 9, 10236. [Google Scholar] [CrossRef] [Green Version]
- Liakopoulos, V.; Jeron, A.; Shah, A.; Bruder, D.; Mertens, P.R.; Gorny, X. Hemodialysis-related changes in phenotypical features of monocytes. Sci. Rep. 2018, 8, 13964. [Google Scholar] [CrossRef] [Green Version]
- Brandt, S.; Ewert, L.; Scurt, F.G.; Reichardt, C.; Lindquist, J.A.; Gorny, X.; Isermann, B.; Mertens, P.R. Altered monocytic phenotypes are linked with systemic inflammation and may be linked to mortality in dialysis patients. Sci. Rep. 2019, 9, 19103. [Google Scholar] [CrossRef] [Green Version]
- Betjes, M.G. Immune cell dysfunction and inflammation in end-stage renal disease. Nat. Rev. Nephrol. 2013, 9, 255–265. [Google Scholar] [CrossRef]
- Fukushi, T.; Yamamoto, T.; Yoshida, M.; Fujikura, E.; Miyazaki, M.; Nakayama, M. Enhanced neutrophil apoptosis accompanying myeloperoxidase release during hemodialysis. Sci. Rep. 2020, 10, 21747. [Google Scholar] [CrossRef]
- Song, J.W.; Zhang, C.; Fan, X.; Meng, F.P.; Xu, Z.; Xia, P.; Cao, W.J.; Yang, T.; Dai, X.P.; Wang, S.Y.; et al. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat. Commun. 2020, 11, 3410. [Google Scholar] [CrossRef]
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020, 369, eabc8511. [Google Scholar] [CrossRef]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, Y.; Ling, Y.; Lu, G.; Liu, F.; Yi, Z.; Jia, X.; Wu, M.; Shi, B.; Xu, S.; et al. Viral and host factors related to the clinical outcome of COVID-19. Nature 2020, 583, 437–440. [Google Scholar] [CrossRef]
- Utrero-Rico, A.; Gonzalez-Cuadrado, C.; Chivite-Lacaba, M.; Cabrera-Marante, O.; Laguna-Goya, R.; Almendro-Vazquez, P.; Diaz-Pedroche, C.; Ruiz-Ruigomez, M.; Lalueza, A.; Folgueira, M.D.; et al. Alterations in Circulating Monocytes Predict COVID-19 Severity and Include Chromatin Modifications Still Detectable Six Months after Recovery. Biomedicines 2021, 9, 1253. [Google Scholar] [CrossRef]
- Betjes, M.G.H. Uremia-Associated Immunological Aging and Severity of COVID-19 Infection. Front. Med. 2021, 8, 675573. [Google Scholar] [CrossRef]
- Gisby, J.; Clarke, C.L.; Medjeral-Thomas, N.; Malik, T.H.; Papadaki, A.; Mortimer, P.M.; Buang, N.B.; Lewis, S.; Pereira, M.; Toulza, F.; et al. Longitudinal proteomic profiling of dialysis patients with COVID-19 reveals markers of severity and predictors of death. Elife 2021, 10, e64827. [Google Scholar] [CrossRef]
- El Karoui, K.; De Vriese, A.S. COVID-19 in dialysis: Clinical impact, immune response, prevention, and treatment. Kidney Int. 2022, 101, 883–894. [Google Scholar] [CrossRef]
- Cobo, G.; Lindholm, B.; Stenvinkel, P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol. Dial. Transplant. 2018, 33, iii35–iii40. [Google Scholar] [CrossRef]
- Laguna-Goya, R.; Utrero-Rico, A.; Talayero, P.; Lasa-Lazaro, M.; Ramirez-Fernandez, A.; Naranjo, L.; Segura-Tudela, A.; Cabrera-Marante, O.; Rodriguez de Frias, E.; Garcia-Garcia, R.; et al. IL-6-based mortality risk model for hospitalized patients with COVID-19. J. Allergy Clin. Immunol. 2020, 146, 799–807.e9. [Google Scholar] [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Alberici, F.; Affatato, S.; Moratto, D.; Mescia, F.; Delbarba, E.; Guerini, A.; Tedesco, M.; Burbelo, P.D.; Zani, R.; Castagna, I.; et al. SARS-CoV-2 infection in dialysis and kidney transplant patients: Immunological and serological response. J. Nephrol. 2022, 35, 745–759. [Google Scholar] [CrossRef]
- Xiang, F.F.; Zhu, J.M.; Cao, X.S.; Shen, B.; Zou, J.Z.; Liu, Z.H.; Zhang, H.; Teng, J.; Liu, H.; Ding, X.Q. Lymphocyte depletion and subset alteration correlate to renal function in chronic kidney disease patients. Ren. Fail. 2016, 38, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Park, S.S.; Kim, T.Y.; Lee, D.G.; Kim, D.W. Lymphopenia as a Biological Predictor of Outcomes in COVID-19 Patients: A Nationwide Cohort Study. Cancers 2021, 13, 471. [Google Scholar] [CrossRef]
- Lano, G.; Braconnier, A.; Bataille, S.; Cavaille, G.; Moussi-Frances, J.; Gondouin, B.; Bindi, P.; Nakhla, M.; Mansour, J.; Halin, P.; et al. Risk factors for severity of COVID-19 in chronic dialysis patients from a multicentre French cohort. Clin. Kidney J. 2020, 13, 878–888. [Google Scholar] [CrossRef]
- Sallusto, F.; Geginat, J.; Lanzavecchia, A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu. Rev. Immunol. 2004, 22, 745–763. [Google Scholar] [CrossRef]
- Schulte-Schrepping, J.; Reusch, N.; Paclik, D.; Bassler, K.; Schlickeiser, S.; Zhang, B.; Kramer, B.; Krammer, T.; Brumhard, S.; Bonaguro, L.; et al. Severe COVID-19 Is Marked by a Dysregulated Myeloid Cell Compartment. Cell 2020, 182, 1419–1440.e23. [Google Scholar] [CrossRef]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef]
- Nockher, W.A.; Scherberich, J.E. Expanded CD14+ CD16+ monocyte subpopulation in patients with acute and chronic infections undergoing hemodialysis. Infect. Immun. 1998, 66, 2782–2790. [Google Scholar] [CrossRef]
- Girndt, M.; Sester, U.; Kaul, H.; Kohler, H. Production of proinflammatory and regulatory monokines in hemodialysis patients shown at a single-cell level. J. Am. Soc. Nephrol. 1998, 9, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Kapellos, T.S.; Bonaguro, L.; Gemund, I.; Reusch, N.; Saglam, A.; Hinkley, E.R.; Schultze, J.L. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front. Immunol. 2019, 10, 2035. [Google Scholar] [CrossRef] [Green Version]
- Wen, W.; Su, W.; Tang, H.; Le, W.; Zhang, X.; Zheng, Y.; Liu, X.; Xie, L.; Li, J.; Ye, J.; et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Cerrillo, I.; Landete, P.; Aldave, B.; Sanchez-Alonso, S.; Sanchez-Azofra, A.; Marcos-Jimenez, A.; Avalos, E.; Alcaraz-Serna, A.; de Los Santos, I.; Mateu-Albero, T.; et al. COVID-19 severity associates with pulmonary redistribution of CD1c+ DCs and inflammatory transitional and nonclassical monocytes. J. Clin. Investig. 2020, 130, 6290–6300. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000.e3. [Google Scholar] [CrossRef] [PubMed]
- Sepe, V.; Libetta, C.; Gregorini, M.; Rampino, T. The innate immune system in human kidney inflammaging. J. Nephrol. 2022, 35, 381–395. [Google Scholar] [CrossRef]
- Malaponte, G.; Bevelacqua, V.; Fatuzzo, P.; Rapisarda, F.; Emmanuele, G.; Travali, S.; Mazzarino, M.C. IL-1beta, TNF-alpha and IL-6 release from monocytes in haemodialysis patients in relation to dialytic age. Nephrol. Dial. Transplant. 2002, 17, 1964–1970. [Google Scholar] [CrossRef] [Green Version]
- Ashby, D.R.; Caplin, B.; Corbett, R.W.; Asgari, E.; Kumar, N.; Sarnowski, A.; Hull, R.; Makanjuola, D.; Cole, N.; Chen, J.; et al. Outcome and effect of vaccination in SARS-CoV-2 Omicron infection in hemodialysis patients: A cohort study. Nephrol. Dial. Transplant. 2022, 37, 1944–1950. [Google Scholar] [CrossRef]





| Characteristics | HD (N = 5) | HD + COVID-19 (N = 8) | p-Values |
|---|---|---|---|
| Age (Median, [IQR]) | 54.2 [(50–58) | 55 (48–70) | 0.5 |
| Sex, male (n, %) | 3 (60%) | 6 (75%) | 0.6 |
| Active smoking (yes) | 2 (40%) | 2 (25%) | 0.5 |
| HD time, months (Median, IQR) | 31 (31–36) | 44 (29–47) | 0.5 |
| Use of EPO (n, %) | 5 (100%) | 5 (62.5%) | 0.2 |
| Previous kidney transplantation (n, %) | 1 (20%) | 1 (12.5%) | >0.9 |
| Mean arterial pressure (Median, IQR) | 103.7 (97.7–106) | 88 (81.6–95.1) | 0.1 |
| Comorbidities (n, %) | |||
| Obesity | 1 (12.5%) | ||
| Hypertension | 5 (100%) | 7 (87.5%) | 0.5 |
| Diabetes mellitus | 3 (37.5%) | ||
| Ischemic heart disease | 2 (40%) | 3 (37.5%) | >0.9 |
| Dyslipidemia | 2 (40%) | 2 (25%) | >0.9 |
| Cause of end-stage renal disease (n, %) | |||
| Diabetic nephropathy | 2 (25%) | ||
| Hydronephrosis | 2 (40%) | 1 (12.5%) | 0.5 |
| Hypertensive nephrosclerosis | 2 (25%) | ||
| Hemolytic-uraemic syndrome | 1 (20%) | 1 (12.5%) | >0.9 |
| IgA nephropathy | 1 (20%) | 2 (25%) | >0.9 |
| Membranoproliferative glomerulonephritis | 1 (20%) | ||
| Laboratory parameters on test day (Median, [IQR]) | |||
| Serum creatinine, mg/dL | 9.7 (6.2–10.1) | 7.5 (5.5–9.4) | >0.9 |
| C-reactive protein, mg/dL | 0.3 (0.1–0.4) | 3.6 (0.8–3.9) | * |
| Albumin, g/dL | 4.3 (4.1–4.4) | 3.5 (3.3–4.4) | 0.1 |
| ALT, U/L | 10 (8–11) | 16 (9–32.8) | 0.2 |
| AST, U/L | 10 (10–11) | 20.5 (16.3–26.8) | * |
| Platelets, 103 cell/µL | 125 (116.8–149.3) | 183 (147.8–205.3) | 0.2 |
| Neutrophils, 103 cell/µL | 3.5 (3–4.4) | 5.1 (4.1–5.5) | 0.5 |
| Lymphocytes, 103 cell/µL | 1.1 (1–1.3) | 0.6 (0.4–1.1) | 0.1 |
| Monocytes, 103 cell/µL | 0.6 (0.4–0.7) | 0.5 (0.3–0.6) | 0.6 |
| Fribrinogen, ng/mL | 493 (406–495) | 545 (481–662.8) | 0.1 |
| D-dimer, ng/mL | 336 (247–660) | 906 (467–1300) | 0.4 |
| IL6 a, pg/mL | 1.34 (0.2–2) | 5.9 (1.93–13.9) | 0.06 |
| LDH, U/L | 227.5 (215.8–239.2) | 290 (252.5–306) | 0.6 |
| Saturation O2, % (Median, [IQR]) | 98 (98–100) | ||
| Temperature, °C (Median, [IQR]) | 38 (36.5–38.6) | ||
| COVID-19 treatment on test day (n, %) | |||
| Hydroxychloroquine | 1 (12.5%) | ||
| Antibiotic (Amoxicillin/clavulanic acid, azithromycin, cephalosporin or carbapenem) | 5 (62.5%) | ||
| Corticosteroids | 2 (25%) | ||
| Tozilizumab | 1 (12.5%) | ||
| Anticoagulation | 5 (62.5%) | ||
| Death (n, %) | 2 (25%) | ||
| Days PSO on test day (Median, [IQR]) | 11 (6–14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Cuadrado, C.; Caro-Espada, P.J.; Chivite-Lacaba, M.; Utrero-Rico, A.; Lozano-Yuste, C.; Gutierrez-Solis, E.; Morales, E.; Sandino-Pérez, J.; Gil-Etayo, F.J.; Allende-Martínez, L.; et al. Hemodialysis-Associated Immune Dysregulation in SARS-CoV-2-Infected End-Stage Renal Disease Patients. Int. J. Mol. Sci. 2023, 24, 1712. https://doi.org/10.3390/ijms24021712
González-Cuadrado C, Caro-Espada PJ, Chivite-Lacaba M, Utrero-Rico A, Lozano-Yuste C, Gutierrez-Solis E, Morales E, Sandino-Pérez J, Gil-Etayo FJ, Allende-Martínez L, et al. Hemodialysis-Associated Immune Dysregulation in SARS-CoV-2-Infected End-Stage Renal Disease Patients. International Journal of Molecular Sciences. 2023; 24(2):1712. https://doi.org/10.3390/ijms24021712
Chicago/Turabian StyleGonzález-Cuadrado, Cecilia, Paula Jara Caro-Espada, Marta Chivite-Lacaba, Alberto Utrero-Rico, Claudia Lozano-Yuste, Elena Gutierrez-Solis, Enrique Morales, Justo Sandino-Pérez, Francisco Javier Gil-Etayo, Luis Allende-Martínez, and et al. 2023. "Hemodialysis-Associated Immune Dysregulation in SARS-CoV-2-Infected End-Stage Renal Disease Patients" International Journal of Molecular Sciences 24, no. 2: 1712. https://doi.org/10.3390/ijms24021712