A Novel Indole Derivative with Superior Photophysical Performance for Fluorescent Probe, pH-Sensing, and Logic Gates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Solvatochromism
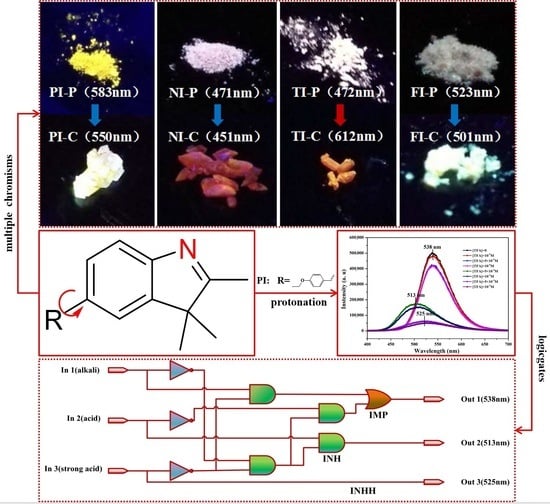
2.3. Solid State Luminescence Property and Multiple Chromism (MC) Effect
2.4. Electronic Structure
2.5. pH-Sensing
2.6. Fluorescent Paper
2.7. Logic Gate Application
3. Materials and Methods
3.1. Materials and Measurements
3.2. Synthetic Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, M.H.; Han, J.H.; Kwon, P.S.; Bhuniya, S.; Kim, J.Y.; Sessler, J.L.; Kang, C.; Kim, J.S. Hepatocyte-targeting single galactose-appended naphthalimide: A tool for intracellular thiol imaging in vivo. J. Am. Chem. Soc. 2012, 134, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Zhong, K.L.; Liu, H.L.; Jin, S.; Chen, X. Design of fluorescence turn-on sensors with novel response mechanism based on C=N isomerization. Dyes Pigm. 2021, 195, 109714. [Google Scholar] [CrossRef]
- Kothavale, S.; Jadhav, A.G.; Sekar, N. Deep red emitting triphenylamine based coumarin-rhodamine hybrids with large Stokes shift and viscosity sensing: Synthesis, photophysical properties and DFT studies of their spirocyclic and open forms. Dyes Pigm. 2017, 137, 329–341. [Google Scholar] [CrossRef]
- Xue, Y.; Xia, X.; Yu, B.; Luo, X.; Cai, N.; Long, S.; Yu, F. A green and facile method for the preparation of a pH-responsive alginate nanogel for subcellular delivery of doxorubicin. RSC Adv. 2015, 5, 73416. [Google Scholar] [CrossRef]
- Yoon, S.A.; Lee, J.; Lee, M.H. A ratiometric fluorescent probe for Zn2+ based on pyrene-appended naphthalimide-dipicolylamine. Sens. Actuator B 2018, 258, 50–55. [Google Scholar] [CrossRef]
- Ma, B.; Wu, S.; Zeng, F. Reusable polymer film chemosensor for ratiometric fluorescence sensing in aqueous media. Sens. Actuator B 2010, 145, 451. [Google Scholar] [CrossRef]
- Thomas, S.; Joly, G.; Swager, T. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339. [Google Scholar] [CrossRef]
- Samuel, I.; Turnbull, G. Amplified spontaneous emission and lasing properties of bisfluorene-cored dendrimers. Chem. Rev. 2007, 107, 1272. [Google Scholar] [CrossRef]
- Jin, Q.; Hu, Y.; Shen, J.; Li, B.; Kan, C. A novel 1, 8-naphthalimide green fluorescent dye and its corresponding intrinsically fluorescent polyurethane latexes. J. Coat. Technol. Res. 2017, 14, 571–582. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.; Tang, B. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361. [Google Scholar] [CrossRef]
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-induced emission: The whole is more brilliant than the parts. Adv. Mater. 2014, 26, 5429. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, Z.; Meng, X.; Ma, Z.; Xu, Z.; Ma, Y.; Jia, X. A mechanochromic single crystal: Turning two color changes into a tricolored switch. Angew. Chem. Int. Ed. 2016, 55, 519. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, J.; Deng, H.; Zhao, E.; Shen, B.; Lam, J.W.Y.; Wong, K.S.; Wu, H.; Li, B.S.; Tang, B.Z. Aggregation-induced chirality, circularly polarized luminescence, and helical self-assembly of a leucine-containing AIE luminogen. J. Mater. Chem. C 2015, 3, 2399. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.-F.; Aldred, M.P.; Gong, W.-L.; Li, C.; Zhu, M.-Q. Utilising tetraphenylethene as a dual activator for intramolecular charge transfer and aggregation induced emission. Chem. Commun. 2012, 48, 7711. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mei, J.; Hu, R.; Sun, J.Z.; Qin, A.; Tang, B.Z. Aggregation-induced emission, E/Z isomerization, self-organization, and multiple chromisms of pure stereoisomers of a tetraphenylethene-cored luminogen. J. Am. Chem. Soc. 2012, 134, 9956. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, C.Y.Y.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. A photostable AIE luminogen with near infrared emission for monitoring morphological change of plasma membrane. J. Mater. Chem. B 2018, 6, 1501. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Liu, H.; Jin, S.; Wu, G. Interconnected magnetic carbon@NixCo1-xFe2O4 nanospheres with core-shell structure: An efficient and thin electromagnetic wave absorber. J. Colloid. Inter. Sci. 2022, 606, 526–536. [Google Scholar] [CrossRef]
- Mao, Y.; Guan, Y.; Luo, D.; Zheng, Q.; Feng, X.; Wang, X. Investigation of a homogeneous activating ozonation method in the rinsing procedure of cotton fabric dyed with reactive dye. Color. Technol. 2011, 127, 256–267. [Google Scholar] [CrossRef]
- Khopkar, S.; Shankarling, G. Synthesis, photophysical properties and applications of NIR absorbing unsymmetrical squaraines: A review. Dye Pigm. 2019, 170, 107645. [Google Scholar] [CrossRef]
- Ali, S.S.; Gangopadhyay, A.; Maiti, K.; Mondal, S.; Pramanik, A.K.; Guria, U.N.; Uddin, M.R.; Mandal, S.; Mandal, D.; Mahapatra, A.K. A chromogenic and ratiometric fluorogenic probe for rapid detection of a nerve agent simulant DCP based on a hybrid hydroxynaphthalene-hemicyanine dye. Org. Biomol. Chem. 2017, 15, 5959–5967. [Google Scholar] [CrossRef] [PubMed]
- Pattaweepaiboon, S.; Nanok, T.; Kaewchangwat, N.; Suttisintong, K.; Sirisaksoontorn, W. Colorimetric detection of Hg2+ and CH3Hg+ by a novel spirooxazine derivative as a highly sensitive and selective probe. Dye. Pigment. 2021, 186, 108996. [Google Scholar] [CrossRef]
- Zhang, Z.; Kwok, R.T.K.; Yu, Y.; Tang, B.Z.; Ng, K.M. Sensitive and specific detection of l-lactate using an AIE-active fluorophore. ACS Appl. Mater. Inter. 2017, 9, 38153. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Li, P.; Tang, B. Small molecular fluorescent probes for imaging of viscosity in living biosystems. Chem. A Euro. J. 2021, 27, 6880–6898. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Liu, J.; Zhao, J.; Zhao, B.-X.; Miao, J.-Y. Highly selective and sensitive pH-responsive fluorescent probe in living Hela and HUVEC cells. Sens. Actuator B 2013, 177, 956. [Google Scholar] [CrossRef]
- Irani, M.M.; Nourmohammadian, F.; Bastani, S.; Najafi, F. Development of diketopyrrolopyrrole fluorescent dyes with different alkyl chain length in organic film: Photophysical and rheological properties. Prog. Org. Coat. 2019, 131, 176–190. [Google Scholar] [CrossRef]
- Yan, H.; Meng, X.L.; Li, B.Y.; Ge, S.S.; Lu, Y. Design, synthesis, photophysical properties and pH-sensing application of pyrimidine-phthalimide derivatives. J. Mater. Chem. C 2017, 5, 10589–10599. [Google Scholar] [CrossRef]
- Jin, H.; Yang, M.; Sun, Z.; Gui, R. Ratiometric two-photon fluorescence probes for sensing, imaging and biomedicine applications at living cell and small animal levels. Coord. Chem. Rev. 2021, 446, 214114. [Google Scholar] [CrossRef]
- Cao, D.; Liu, Z.; Li, G. A trivalent organoboron compound as one and two-photon fluorescent chemosensor for fluoride anion. Sens. Actuator B 2008, 133, 489. [Google Scholar] [CrossRef]
- Cao, D.; Liu, Z.; Li, G.; Liu, G.; Zhang, G. Synthesis and blue-violet two-photon excited fluorescence of a new organoboron compound. J. Mol. Struct. 2008, 874, 46. [Google Scholar] [CrossRef]
- Song, X.; Johnson, A.; Foley, J. 7-Azabicyclo [2.2. 1] heptane as a unique and effective dialkylamino auxochrome moiety: Demonstration in a fluorescent rhodamine dye. J. Am. Chem. Soc. 2008, 130, 17653. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhan, X.-Q.; Bian, Q.-N.; Zhang, X.-J. Advances in modifying fluorescein and rhodamine fluorophores as fluorescent chemosensors. Chem. Commun. 2013, 49, 429. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, Q.; Feng, W.; Li, F. Luminescent chemodosimeters for bioimaging. Chem. Rev. 2013, 113, 192. [Google Scholar] [CrossRef]
- Fan, L.; Fu, Y.-J.; Liu, Q.-L.; Lu, D.-T.; Dong, C.; Shuang, S.-M. Novel far-visible and near-infrared pH probes based on styrylcyanine for imaging intracellular pH in live cells. Chem. Commun. 2012, 48, 11202. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhang, W.; Li, P.; Xing, Y.; Tong, L.; Ma, J.; Tang, B. Cu2+-selective naked-eye and fluorescent probe: Its crystal structure and application in bioimaging. Analyst 2009, 9, 1826. [Google Scholar] [CrossRef]
- Cao, X.; Lin, W.; Yu, Q.; Wang, J. Ratiometric sensing of fluoride anions based on a BODIPY-coumarin platform. Org. Lett. 2011, 13, 6098. [Google Scholar] [CrossRef]
- Hassan, H.B. Density function theory B3LYP/6-31G** calculation of geometry optimization and energies of donor-bridge-acceptor molecular system. Int. J. Curr. Eng. Technol. 2014, 4, 2342–2345. [Google Scholar]
- Lazrak, M.; Toufik, H.; Bouzzine, S.M.; Lamchouri, F. Bridge effect on the charge transfer and optoelectronic properties of triphenylamine-based organic dye sensitized solar cells: Theoretical approach. Res. Chem. Intermed. 2020, 46, 3961–3978. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, Z.; Fang, Q. Donor-and-acceptor substituted truxenes as multifunctional fluorescent probes. J. Org. Chem. 2007, 72, 7915. [Google Scholar] [CrossRef]
- Liu, X.; Bai, D.; Wang, S. Charge-transfer emission in nonplanar three-coordinate organoboron compounds for fluorescent sensing of fluoride. Angew. Chem. Int. Ed. 2006, 45, 5475. [Google Scholar] [CrossRef]
- Shi, H.-P.; Dai, J.-X.; Xu, L.; Shi, L.-W.; Fang, L.; Shuang, S.-M.; Dong, C. A boron-containing carbazole dimer: Synthesis, photophysical properties and sensing properties. Org. Biomol. Chem. 2012, 10, 3852. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Baumgarten, M.; Müllen, K. Mesitylboron-substituted ladder-type pentaphenylenes: Charge-transfer, electronic communication, and sensing properties. J. Am. Chem. Soc. 2008, 130, 12477. [Google Scholar] [CrossRef] [PubMed]















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.-L.; Zhan, K.; Zhong, K.-L.; Chen, X.-L.; Xia, X.-H. A Novel Indole Derivative with Superior Photophysical Performance for Fluorescent Probe, pH-Sensing, and Logic Gates. Int. J. Mol. Sci. 2023, 24, 1711. https://doi.org/10.3390/ijms24021711
Liu H-L, Zhan K, Zhong K-L, Chen X-L, Xia X-H. A Novel Indole Derivative with Superior Photophysical Performance for Fluorescent Probe, pH-Sensing, and Logic Gates. International Journal of Molecular Sciences. 2023; 24(2):1711. https://doi.org/10.3390/ijms24021711
Chicago/Turabian StyleLiu, Hai-Ling, Kan Zhan, Kai-Liang Zhong, Xing-Liang Chen, and Xing-Hua Xia. 2023. "A Novel Indole Derivative with Superior Photophysical Performance for Fluorescent Probe, pH-Sensing, and Logic Gates" International Journal of Molecular Sciences 24, no. 2: 1711. https://doi.org/10.3390/ijms24021711