Tiliroside Combined with Anti-MUC1 Monoclonal Antibody as Promising Anti-Cancer Strategy in AGS Cancer Cells
Abstract
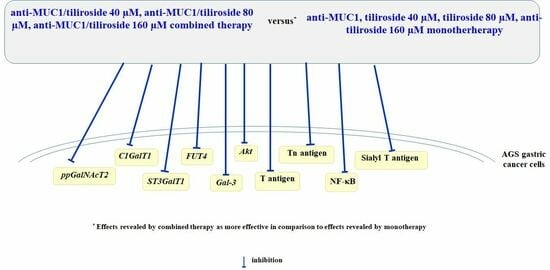
:1. Introduction
2. Results
2.1. Effects of Tiliroside and Anti-MUC1 mAb on the Viability of AGS Gastric Cancer Cells
2.2. Effects of Anti-MUC1 mAb on MUC1 Expression
2.3. Effects of Tiliroside and Anti-MUC1 mAb on Tn Antigen Expression
2.4. Effects of Tiliroside and Anti-MUC1 mAb on T, sialyl T antigens Expression, and Enzymes Responsible for Their Formation
2.5. Effects of Tiliroside and Anti-MUC1 mAb on Fucosylated Antigen Expression and the Enzyme Responsible for Its Formation
2.6. Effects of Tiliroside and Anti-MUC1 mAb on Galectin-3 Expression
2.7. Effects of Tiliroside and Anti-MUC1 mAb on NF-κB Expression
2.8. Effects of Tiliroside and Anti-MUC1 mAb on Akt Expression
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Viability Test
4.3. RNA Isolation and Quantitative Real-Time PCR
4.4. ELISA Tests
4.5. Western Blotting
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petryszyn, P.; Chapell, N.; Matysiak-Budnik, T. Gastric cancer: Where are we heading? Dig. Dis. 2020, 38, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Chen, S.T.; Kuo, T.C.; Lin, T.C.; Lin, M.C.; Huang, J.; Huang, J.S.; Hsu, C.L.; Juan, H.F.; Lee, P.H.; et al. C1GALT1 is associated with poor survival and promotes soluble Ephrin A1-mediated cell migration through activation of EPHA2 in gastric cancer. Oncogene 2020, 39, 2724–2740. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Selvaduray, K.R.; Radhakrishnan, A.K. Bioactive compounds: Natural defense against cancer? Biomolecules 2019, 9, 758. [Google Scholar] [CrossRef] [PubMed]
- Scaria, B.; Sood, S.; Raad, C.; Khanafer, J.; Jayachandiran, R.; Pupulin, A.; Grewal, S.; Okoko, M.; Arora, M.; Miles, L.; et al. Natural health products (NHP’s) and natural compounds as therapeutic agents for the treatment of cancer; mechanisms of anti-cancer activity of natural compounds and overall trends. Int. J. Mol. Sci. 2020, 21, 8480. [Google Scholar] [CrossRef]
- Maiuolo, J.; Musolino, V.; Gliozzi, M.; Carresi, C.; Oppedisano, F.; Nucera, S.; Scarano, F.; Scicchitano, M.; Guarnieri, L.; Bosco, F.; et al. The employment of genera Vaccinium, Citrus, Olea, and Cynara polyphenols for the reduction of selected anti-cancer drug side effects. Nutrients 2022, 14, 1574. [Google Scholar] [CrossRef]
- Hazafa, A.; Rehman, K.U.; Jahan, N.; Jabeen, Z. The role of polyphenol (flavonoids) compounds in the treatment of cancer cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef]
- Ramos, S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signaling pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef]
- Arts, I.C.W.; Jacobs, D.R., Jr.; Gross, M.; Harnack, L.J.; Folsom, A.R. Dietary catechins and cancer incidence among postmenopausal women: The Iowa Women’s Health Study (United States). Cancer Causes Control 2002, 13, 373–382. [Google Scholar] [CrossRef]
- Luhata, L.P.; Luhata, W.B. Tiliroside: Biosynthesis, bioavailability and structure activity relationship (SAR)—A review. J. Phytopharmacol. 2017, 6, 343–348. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Locatelli, M.; Granica, S.; Cacciagrano, F.; Tomczyk, M. A review on the dietary flavonoid tiliroside. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1395–1421. [Google Scholar] [CrossRef]
- Sukumar, D.; Aparna, C. Phytochemical and antibacterial effect of Delonix elata. Int. J. Sci. Res. 2014, 3, 56–57. [Google Scholar] [CrossRef]
- Han, R.; Yang, H.; Lu, L.; Lin, L. Tiliroside as a CAXII inhibitor suppresses liver cancer development and modulates E2Fs/Caspase-3 axis. Sci. Rep. 2021, 11, 8626. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Yang, H.; Ling, C.; Lu, L. Tiliroside suppresses triple-negative breast cancer as a multifunctional CAXII inhibitor. Cancer Cell Int. 2022, 22, 368. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lu, T.; Liu, M.; Yuan, X.; Li, D.; Zhang, J.; Zhou, L.; Xu, M. Tiliroside targets TBK1 to induce ferroptosis and sensitize hepatocellular carcinoma to sorafenib. Phytomedicine 2023, 111, 154668. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhao, J.F.; Wang, Y.M.; Wu, X.I.; Ye, L. Tiliroside induces ferroptosis to repress the development of triple-negative breast cancer cells. Tissue Cell 2023, 83, 102116. [Google Scholar] [CrossRef]
- Augustynowicz, D.; Lemieszek, M.K.; Strawa, J.W.; Wiater, A.; Tomczyk, M. Phytochemical profiling of extracts from rare Potentilla species and evaluation of their anticancer potential. Int. J. Mol. Sci. 2023, 24, 4836. [Google Scholar] [CrossRef]
- Jin, X.; Song, S.; Wang, J.; Zhang, Q.; Qiu, F.; Zhao, F. Tiliroside, the major component of Agrimonia pilosa Ledeb ethanol extract, inhibits MAPK/JNK/p38-mediated inflammation in lipopolysaccharide-activated RAW 2647 macrophages. Exp. Ther. Med. 2016, 12, 499–505. [Google Scholar] [CrossRef]
- Freitas, D.; Campos, D.; Gomes, J.; Pinto, F.; Macedo, J.A.; Matos, R.; Mereiter, S.; Pinto, M.T.; Polonia, A.; Gartner, F.; et al. O-glycans truncation modulates gastric cancer cell signaling and transcription leading to a more aggressive phenotype. EBioMedicine 2019, 40, 349–362. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Z.; Zhang, S.; Zhu, P.; Ko, J.K.S.; Yung, K.K.L. MUC1: Structure, function, and clinic application in epithelial cancers. Int. J. Mol. Sci. 2021, 22, 6567. [Google Scholar] [CrossRef]
- Nath, S.; Mukherjee, P. Muc1: A multifaceted oncoprotein with a key role in cancer progression. Trends Mol. Med. 2014, 20, 332–342. [Google Scholar] [CrossRef]
- Van Putten, J.P.M.; Strijbis, K. Transmembrane mucins: Signaling receptors at the intersection of inflammation and cancer. J. Innate Immun. 2017, 9, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Kufe, D.W. MUC1-C in chronic inflammation and carcinogenesis; emergence as a target for cancer treatment. Carcinogenesis 2020, 41, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Qing, L.; Li, Q.; Dong, Z. MUC1: An emerging target in cancer treatment and diagnosis. Bull. Cancer 2022, 109, 1202–1216. [Google Scholar] [CrossRef] [PubMed]
- Cascio, S.; Finn, O.J. Intra- and extra-cellular events related to altered glycosylation of MUC1 promote chronic inflammation, tumor progression, invasion, and metastasis. Biomolecules 2016, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Chia, J.; Goh, G.; Bard, F. Short O-GalNAc glycans: Regulation and role in tumor development and clinical perspectives. Biochim. Biophys. Acta 2016, 1660, 1623–1639. [Google Scholar] [CrossRef]
- Hossain, F.; Andreana, P.R. Developments in carbohydrate-based cancer therapies. Pharmaceticals 2019, 12, 84. [Google Scholar] [CrossRef]
- Feng, D.; Shaikh, A.S.; Wang, F. Recent advance in tumor-associated carbohydrate antigens (TACAs)-based antitumor vaccines. ACS Chem. Biol. 2016, 11, 850–863. [Google Scholar] [CrossRef]
- Radziejewska, I.; Supruniuk, K.; Tomczyk, M.; Izdebska, W.; Borzym-Kluczyk, M.; Bielawska, A.; Bielawski, K.; Galicka, A. p-Coumaric acid, kaempferol, astragalin and tiliroside influence the expression of glycoforms in AGS gastric cancer cells. Int. J. Mol. Sci. 2022, 23, 8602. [Google Scholar] [CrossRef]
- Radziejewska, I.; Supruniuk, K.; Bielawska, A. Anti-cancer effect of combined action of anti-MUC1 and rosmarinic acid in AGS gastric cancer cells. Eur. J. Pharmacol. 2021, 902, 174119. [Google Scholar] [CrossRef]
- Divya, T.; Rathinavel, A.K.; Radhakrishnan, P. Altered glycosylation in cancer: A promising target for biomarkers and therapeutics. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188464. [Google Scholar]
- Mereiter, S.; Balmana, M.; Campos, D.; Gomes, J.; Reis, C.A. Glycosylation in the era of cancer-targeted therapy: Where are we going? Cancer Cell 2019, 36, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.P.; Mandel, U.; Clausen, H.; Gerken, T.A.; Fritz, T.A.; Tabak, L.A. Control of mucin-type O-glycosylation: A classification of the polypeptide GalNAc-transferase gene family. Glycobiology 2012, 22, 736–756. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Chia, J.; Ros, M.; Hui, K.M.; Saltel, F.; Bard, F. Organelle specific O-glycosylation drives MMP14 activation, tumor growth, and metastasis. Cancer Cell 2017, 32, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Lucena, M.C.; Carvalho-Cruz, P.; Donadio, J.L.; Oliveira, I.A.; de Queiroz, R.M.; Marinho-Carvalho, M.M.; Sola-Penna, M.; de Paula, I.F.; Gondim, K.C.; McComb, M.E.; et al. Epithelial mesenchymal transition induces aberrant glycosylation through hexosamine biosynthetic pathway activation. J. Biol. Chem. 2016, 291, 12917–12929. [Google Scholar] [CrossRef]
- Merikhian, P.; Darvishi, B.; Jaslili, N.; Esmailinejad, R.; Khatibi, A.S.; Kalbolandi, S.M.; Salehi, M.; Mosayebzadeh, M.; Barough, M.S.; Majidzadeh-A, K.; et al. Recombinant nanobody against MUC1 tandem repeats inhibits growth, invasion, metastasis, and vascularization of spontaneous mouse mammary tumors. Mol. Oncol. 2022, 16, 485–507. [Google Scholar] [CrossRef]
- Bose, M.; Mukherjee, P. Potential of anti-MUC1 antibodies as a targeted therapy for gastrointestinal cancers. Vaccines 2020, 8, 659. [Google Scholar] [CrossRef]
- Syrkina, M.S.; Vassetzky, Y.S.; Rubtsov, M.A. Muc1 story: Great expectations, disappointments and the renaissance. Curr. Med. Chem. 2019, 26, 554–563. [Google Scholar] [CrossRef]
- Reynolds, I.S.; Fichtner, M.; McNamara, D.A.; Kay, E.W.; Prehn, J.H.; Burke, J.P. Mucin glycoproteins block apoptosis; promote invasion, proliferation, and migration; and cause chemoresistance through diverse pathways in epithelial cancers. Cancer Metastasis Rev. 2019, 38, 237–257. [Google Scholar] [CrossRef]
- Birrer, M.J.; Moore, K.N.; Betella, I.; Bates, R.C. Antibody-drug conjugate-based therapeutics: State of the science. J. Natl. Cancer Inst. 2019, 111, 538–549. [Google Scholar] [CrossRef]
- Wu, G.; Maharjan, S.; Kim, D.; Kim, J.N.; Park, B.K.; Koh, H.; Moon, K.; Lee, Y.; Kwon, H.J. A novel monoclonal antibody targets mucin1 and attenuates growth in pancreatic cancer model. Int. J. Mol. Sci. 2018, 19, 2004. [Google Scholar] [CrossRef]
- Beckwith, D.M.; Cudic, M. Tumor-associated O-glycans of MUC1: Carriers of the glycol-code and targets for cancer vaccine design. Semin. Immunol. 2020, 47, 101389. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, L.A.M.; Blanas, A.; Zaal, A.; van der Horst, J.C.; Kruijssen, L.J.; O’toole, T.; van Kooyk, Y.; van Vliet, S.J. Tn antigen expression contributes to an immune suppressive microenvironment and drives tumor growth in colorectal cancer. Front. Oncol. 2020, 10, 1622. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Zhao, H.; Wang, Y.; Cai, H.; Xiao, Y.; Zeng, Y.; Chen, H. Tumor-associated antigens: Tn antigen, sTn antigen, and T antigen. HLA 2016, 88, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Luan, X.; Zhang, Y.; Robbe-Masselot, C.; Brockhausen, I.; Gao, Y. The expression and functional analysis of sialyl T-antigen in prostate cancer. Glycoconj. J. 2020, 37, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, H.F.; Pace, K.E.; Cabrera, P.V.; White, R.; Porvari, K.; Kaija, H.; Vihko, P.; Baum, L.G. O-glycosylation regulates LNCaP prostate cancer cell susceptibility to apoptosis induced by galectin-1. Cancer Res. 2007, 67, 6155–6162. [Google Scholar] [CrossRef]
- Mereiter, S.; Balmana, M.; Gomes, J.; Magalhaes, A.; Reis, C.A. Glycomic approaches for the discover of targets in gastrointestinal cancer. Front. Oncol. 2016, 6, 55. [Google Scholar] [CrossRef]
- Blanas, A.; Sahasrabudhe, N.M.; Rodriguez, E.; van Kooyk, Y.; van Vliet, S.J. Fucosylated antigens in cancer: An alliance toward tumor progression, metastasis, and resistance to chemotherapy. Front. Oncol. 2018, 8, 39. [Google Scholar] [CrossRef]
- Radziejewska, I. Galectin-3 and epithelial MUC1 mucin—interactions supporting cancer development. Cancers 2023, 15, 2680. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Subramanian, S.; Mallia, M.B.; Repaka, K.; Kaur, S.; Chandan, R.; Bhardwaj, P.; Dash, A.; Banerjee, R. Multifunctional core-shell glyconanoparticles for galectin-3-targeted, trigger-responsive combination chemotherapy. Biomacromolecules 2020, 21, 2645–2660. [Google Scholar] [CrossRef]
- Sindrewicz, P.; Lian, L.Y.; Yu, L.G. Interaction of the oncofetal Thomsen-Friedenreich antigen with galectins in cancer progression and metastasis. Front. Oncol. 2016, 6, 79. [Google Scholar] [CrossRef]
- Li, M.; Chen, Y.; Liu, F.; Qu, J.Q.; Ren, J.C.; Chai, J.; Tang, C.E. Galectin-3 facilitates the proliferation and migration of nasopharyngeal carcinoma cells via activation of the ERK1/2 and Akt signaling pathways and is positively correlated with the inflammatory state of nasopharyngeal carcinoma. Mol. Med. Rep. 2021, 23, 370. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, O.; Naumann, M. NF-κB signaling in gastric cancer. Toxins 2017, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Bose, M.; Sanders, A.; De, C.; Zhou, R.; Lala, P.; Shwartz, S.; Mitra, B.; Brouwer, C.; Mukherjee, P. Targeting tumor-associated MUC1 overcomes anoikis-resistance in pancreatic cancer. Transl. Res. 2023, 253, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, H.; Hiraki, M.; Kufe, D. MUC1-C activates polycomb repressive complexes and downregulates tumor suppressor genes in human cancer cells. Oncogene 2018, 37, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Grochowski, D.M.; Strawa, J.W.; Granica, S.; Tomczyk, M. Secondary metabolites of Rubus caesius (Rosaceae). Biochem. Syst. Ecol. 2020, 92, 104111. [Google Scholar] [CrossRef]
- Carmichael, J.; Degraff, W.; Gazdar, A.; Minna, J.; Mitchell, J. Evaluation of tetrazolium-based semi-automated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar]
- Towbin, T.; Stachelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef]









| Gene | Forward Primer (5′ → 3′) | Reverse Primer (5′ → 3′) |
|---|---|---|
| MUC1 | TGCCTTGGCTGTCTGTCAGT | GTAGGTATCCCGGGCTGGAA |
| C1GalT1 | AAGCAGGGCTACATGAGTGG | GCATCTCCCCAGTGCTAAGT |
| ST3GalT1 | TCGGCCTGGTTCGATGA | CGCGTTCTGGGCAGTCA |
| FUT4 | AAGCCGTTGAGGCGGTTT | ACAGTTGTGTATGAGATTTGGAAGCT |
| Gal-3 | GCAGACAATTTTTCGCTCCATG | CTGTTGTTCTCATTGAAGCGTG |
| Akt | TCTATGGCGCTGAGATTGTG | CTTAATGTGCCCGTCCTTGT |
| NF-κB | CTGAACCAGGGCATACCTGT | GAGAAGTCCATGTCCGCAAT |
| GAPDH | GTGAACCATGAGAAGTATGACAA | CATGAGTCCTTCCACGATAC |
| Antibody | Clone | Source |
|---|---|---|
| Anti-MUC1; extracellular domain (mouse IgG) | BC2 | Abcam |
| Anti-MUC1; cytoplasmic tail (Armenian hamster IgG) | CT2 | Abcam |
| Anti-NF-κB (mouse IgG) | 5D10D11 | Cell Sign Tech |
| Anti-C1GalT1 (mouse IgG) | F-31 | Santa Cruz |
| Anti-FUT4 (mouse IgG) | A-10 | Santa Cruz |
| Anti-ST3GalIV (mouse IgG) | 1F4 | Santa Cruz |
| Anti-Gal-3 (mouse IgG) | B2C10 | Santa Cruz |
| Anti-β-actin (rabbit IgG) | Sigma | |
| Anti-mouse IgG peroxidase conjugated | Sigma | |
| Anti-rabbit IgG peroxidase conjugated | Sigma | |
| Anti-armenian hamster IgG peroxidase conjugated | Abcam |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radziejewska, I.; Supruniuk, K.; Jakimiuk, K.; Tomczyk, M.; Bielawska, A.; Galicka, A. Tiliroside Combined with Anti-MUC1 Monoclonal Antibody as Promising Anti-Cancer Strategy in AGS Cancer Cells. Int. J. Mol. Sci. 2023, 24, 13036. https://doi.org/10.3390/ijms241713036
Radziejewska I, Supruniuk K, Jakimiuk K, Tomczyk M, Bielawska A, Galicka A. Tiliroside Combined with Anti-MUC1 Monoclonal Antibody as Promising Anti-Cancer Strategy in AGS Cancer Cells. International Journal of Molecular Sciences. 2023; 24(17):13036. https://doi.org/10.3390/ijms241713036
Chicago/Turabian StyleRadziejewska, Iwona, Katarzyna Supruniuk, Katarzyna Jakimiuk, Michał Tomczyk, Anna Bielawska, and Anna Galicka. 2023. "Tiliroside Combined with Anti-MUC1 Monoclonal Antibody as Promising Anti-Cancer Strategy in AGS Cancer Cells" International Journal of Molecular Sciences 24, no. 17: 13036. https://doi.org/10.3390/ijms241713036