Circulating Sphingolipids and Glucose Homeostasis: An Update
Abstract
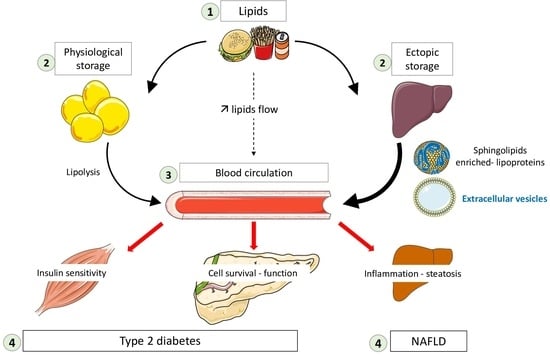
:1. Introduction
2. Ceramides and Lipoproteins
3. Ceramides and Extracellular Vesicles
4. S1P and Lipoproteins
5. S1P and Extracellular Vesicles
6. Conclusions and Open Questions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chew, N.W.S.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E.; et al. The Global Burden of Metabolic Disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428.e3. [Google Scholar] [CrossRef]
- Balakrishnan, V.S. Europe’s Obesity Burden on the Rise: WHO Report. Lancet Diabetes Endocrinol. 2022, 10, 488. [Google Scholar] [CrossRef]
- Lipke, K.; Kubis-Kubiak, A.; Piwowar, A. Molecular Mechanism of Lipotoxicity as an Interesting Aspect in the Development of Pathological States-Current View of Knowledge. Cells 2022, 11, 844. [Google Scholar] [CrossRef] [PubMed]
- Hage Hassan, R.; Bourron, O.; Hajduch, E. Defect of Insulin Signal in Peripheral Tissues: Important Role of Ceramide. World J. Diabetes 2014, 5, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Solis-Herrera, C.; Triplitt, C.; Cersosimo, E.; DeFronzo, R.A.; Feingold, K.R.; Anawalt, B.; Blackman, M.R.; Boyce, A.; Chrousos, G.; Corpas, E.; et al. Pathogenesis of Type 2 Diabetes Mellitus; In Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Lair, B.; Laurens, C.; Van Den Bosch, B.; Moro, C. Novel Insights and Mechanisms of Lipotoxicity-Driven Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 6358. [Google Scholar] [CrossRef]
- Bandet, C.L.; Tan-Chen, S.; Bourron, O.; Stunff, H.L.; Hajduch, E. Sphingolipid Metabolism: New Insight into Ceramide-Induced Lipotoxicity in Muscle Cells. Int. J. Mol. Sci. 2019, 20, 479. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose Tissue and Insulin Resistance in Obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Piché, M.-E.; Tchernof, A.; Després, J.-P. Obesity Phenotypes, Diabetes and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Hajduch, E.; Lachkar, F.; Ferré, P.; Foufelle, F. Roles of Ceramides in Non-Alcoholic Fatty Liver Disease. J. Clin. Med. 2021, 10, 792. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, S. Nonalcoholic Fatty Liver Disease. Endocrinol. Metab. Clin. North. Am. 2023, 52, 149–164. [Google Scholar] [CrossRef]
- Milić, S.; Stimac, D. Nonalcoholic Fatty Liver Disease/Steatohepatitis: Epidemiology, Pathogenesis, Clinical Presentation and Treatment. Dig. Dis. 2012, 30, 158–162. [Google Scholar] [CrossRef]
- Artasensi, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules 2020, 25, 1987. [Google Scholar] [CrossRef] [PubMed]
- Litherland, G.J.; Hajduch, E.; Hundal, H.S. Intracellular Signalling Mechanisms Regulating Glucose Transport in Insulin-Sensitive Tissues (Review). Mol. Membr. Biol. 2001, 18, 195–204. [Google Scholar]
- McGarry, J.D. Banting Lecture 2001: Dysregulation of Fatty Acid Metabolism in the Etiology of Type 2 Diabetes. Diabetes 2002, 51, 7–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodpaster, B.H.; He, J.; Watkins, S.; Kelley, D.E. Skeletal Muscle Lipid Content and Insulin Resistance: Evidence for a Paradox in Endurance-Trained Athletes. J. Clin. Endocrinol. Metab. 2001, 86, 5755–5761. [Google Scholar] [PubMed]
- Jocken, J.W.; Moro, C.; Goossens, G.H.; Hansen, D.; Mairal, A.; Hesselink, M.K.; Langin, D.; van Loon, L.J.; Blaak, E.E. Skeletal Muscle Lipase Content and Activity in Obesity and Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2010, 95, 5449–5453. [Google Scholar] [CrossRef] [Green Version]
- Bellini, L.; Campana, M.; Mahfouz, R.; Carlier, A.; Veret, J.; Magnan, C.; Hajduch, E.; Le, S.H. Targeting Sphingolipid Metabolism in the Treatment of Obesity/Type 2 Diabetes. Expert. Opin. Ther. Targets 2015, 19, 1037–1050. [Google Scholar] [CrossRef]
- Yu, X.-D.; Wang, J.-W. Ceramide de Novo Synthesis in Non-Alcoholic Fatty Liver Disease: Pathogenic Mechanisms and Therapeutic Perspectives. Biochem. Pharmacol. 2022, 202, 115157. [Google Scholar] [CrossRef]
- Maceyka, M.; Spiegel, S. Sphingolipid Metabolites in Inflammatory Disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, M.R.; Jin, H.K.; Bae, J.-S. Diverse Roles of Ceramide in the Progression and Pathogenesis of Alzheimer’s Disease. Biomedicines 2022, 10, 1956. [Google Scholar] [CrossRef]
- Hernández-Bello, F.; Franco, M.; Pérez-Méndez, Ó.; Donis-Maturano, L.; Zarco-Olvera, G.; Bautista-Pérez, R. Sphingolipid Metabolism and Its Relationship with Cardiovascular, Renal and Metabolic Diseases. Arch. Cardiol. Mex. 2023, 93, 88–95. [Google Scholar] [CrossRef]
- Contreras, F.-X.; Ernst, A.M.; Haberkant, P.; Björkholm, P.; Lindahl, E.; Gönen, B.; Tischer, C.; Elofsson, A.; von Heijne, G.; Thiele, C.; et al. Molecular Recognition of a Single Sphingolipid Species by a Protein’s Transmembrane Domain. Nature 2012, 481, 525–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullen, T.D.; Hannun, Y.A.; Obeid, L.M. Ceramide Synthases at the Centre of Sphingolipid Metabolism and Biology. Biochem. J. 2012, 441, 789–802. [Google Scholar] [CrossRef] [Green Version]
- Blachnio-Zabielska, A.U.; Chacinska, M.; Vendelbo, M.H.; Zabielski, P. The Crucial Role of C18-Cer in Fat-Induced Skeletal Muscle Insulin Resistance. Cell Physiol. Biochem. 2016, 40, 1207–1220. [Google Scholar] [CrossRef]
- Tan-Chen, S.; Guitton, J.; Bourron, O.; Le Stunff, H.; Hajduch, E. Sphingolipid Metabolism and Signaling in Skeletal Muscle: From Physiology to Physiopathology. Front. Endocrinol. 2020, 11, 491. [Google Scholar] [CrossRef]
- Raichur, S.; Brunner, B.; Bielohuby, M.; Hansen, G.; Pfenninger, A.; Wang, B.; Bruning, J.C.; Larsen, P.J.; Tennagels, N. The Role of C16:0 Ceramide in the Development of Obesity and Type 2 Diabetes: CerS6 Inhibition as a Novel Therapeutic Approach. Mol. Metab. 2019, 21, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Raichur, S.; Wang, S.T.; Chan, P.W.; Li, Y.; Ching, J.; Chaurasia, B.; Dogra, S.; Ohman, M.K.; Takeda, K.; Sugii, S.; et al. CerS2 Haploinsufficiency Inhibits Beta-Oxidation and Confers Susceptibility to Diet-Induced Steatohepatitis and Insulin Resistance. Cell Metab. 2014, 20, 687–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.W.; Park, W.J.; Kuperman, Y.; Boura-Halfon, S.; Pewzner-Jung, Y.; Futerman, A.H. Ablation of Very Long Acyl Chain Sphingolipids Causes Hepatic Insulin Resistance in Mice Due to Altered Detergent-Resistant Membranes. Hepatology 2013, 57, 525–532. [Google Scholar] [CrossRef]
- Pewzner-Jung, Y.; Park, H.; Laviad, E.L.; Silva, L.C.; Lahiri, S.; Stiban, J.; Erez-Roman, R.; Brügger, B.; Sachsenheimer, T.; Wieland, F.; et al. A Critical Role for Ceramide Synthase 2 in Liver Homeostasis: I. Alterations in Lipid Metabolic Pathways. J. Biol. Chem. 2010, 285, 10902–10910. [Google Scholar] [CrossRef] [Green Version]
- Pewzner-Jung, Y.; Brenner, O.; Braun, S.; Laviad, E.L.; Ben-Dor, S.; Feldmesser, E.; Horn-Saban, S.; Amann-Zalcenstein, D.; Raanan, C.; Berkutzki, T.; et al. A Critical Role for Ceramide Synthase 2 in Liver Homeostasis: II. Insights into Molecular Changes Leading to Hepatopathy. J. Biol. Chem. 2010, 285, 10911–10923. [Google Scholar] [CrossRef] [Green Version]
- Turpin-Nolan, S.M.; Brüning, J.C. The Role of Ceramides in Metabolic Disorders: When Size and Localization Matters. Nat. Rev. Endocrinol. 2020, 16, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, B.; Summers, S.A. Ceramides in Metabolism: Key Lipotoxic Players. Annu. Rev. Physiol. 2021, 83, 303–330. [Google Scholar] [CrossRef]
- Blouin, C.M.; Prado, C.; Takane, K.K.; Lasnier, F.; Garcia-Ocana, A.; Ferre, P.; Dugail, I.; Hajduch, E. Plasma Membrane Subdomain Compartmentalization Contributes to Distinct Mechanisms of Ceramide Action on Insulin Signaling. Diabetes 2010, 59, 600–610. [Google Scholar] [CrossRef] [Green Version]
- Powell, D.J.; Turban, S.; Gray, A.; Hajduch, E.; Hundal, H.S. Intracellular Ceramide Synthesis and Protein Kinase C Zeta Activation Play an Essential Role in Palmitate-Induced Insulin Resistance in Rat L6 Skeletal Muscle Cells. Biochem. J. 2004, 382, 619–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, D.J.; Hajduch, E.; Kular, G.; Hundal, H.S. Ceramide Disables 3-Phosphoinositide Binding to the Pleckstrin Homology Domain of Protein Kinase B (PKB)/Akt by a PKCzeta-Dependent Mechanism. Mol. Cell Biol. 2003, 23, 7794–7808. [Google Scholar] [CrossRef] [Green Version]
- Hage Hassan, R.; Pacheco de Sousa, A.C.; Mahfouz, R.; Hainault, I.; Blachnio-Zabielska, A.; Bourron, O.; Koskas, F.; Gorski, J.; Ferre, P.; Foufelle, F.; et al. Sustained Action of Ceramide on the Insulin Signaling Pathway in Muscle Cells: Implication of the Double-Stranded RNA-Aactivated Protein Kinase. J. Biol. Chem. 2016, 291, 3019–3029. [Google Scholar] [CrossRef] [Green Version]
- Fayyaz, S.; Japtok, L.; Kleuser, B. Divergent Role of Sphingosine 1-Phosphate on Insulin Resistance. Cell Physiol. Biochem. 2014, 34, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Taha, T.A.; Hannun, Y.A.; Obeid, L.M. Sphingosine Kinase: Biochemical and Cellular Regulation and Role in Disease. J. Biochem. Mol. Biol. 2006, 39, 113–131. [Google Scholar] [CrossRef]
- Haass, N.K.; Nassif, N.; McGowan, E.M. Switching the Sphingolipid Rheostat in the Treatment of Diabetes and Cancer Comorbidity from a Problem to an Advantage. Biomed. Res. Int. 2015, 2015, 165105. [Google Scholar] [CrossRef] [Green Version]
- Guitton, J.; Bandet, C.L.; Mariko, M.L.; Tan-Chen, S.; Bourron, O.; Benomar, Y.; Hajduch, E.; Le Stunff, H. Sphingosine-1-Phosphate Metabolism in the Regulation of Obesity/Type 2 Diabetes. Cells 2020, 9, 1682. [Google Scholar] [CrossRef]
- Wigger, D.; Schumacher, F.; Schneider-Schaulies, S.; Kleuser, B. Sphingosine 1-Phosphate Metabolism and Insulin Signaling. Cell Signal 2021, 82, 109959. [Google Scholar] [CrossRef]
- Haus, J.M.; Kashyap, S.R.; Kasumov, T.; Zhang, R.; Kelly, K.R.; DeFronzo, R.A.; Kirwan, J.P. Plasma Ceramides Are Elevated in Obese Subjects with Type 2 Diabetes and Correlate with the Severity of Insulin Resistance. Diabetes 2009, 58, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Neeland, I.J.; Singh, S.; McGuire, D.K.; Vega, G.L.; Roddy, T.; Reilly, D.F.; Castro-Perez, J.; Kozlitina, J.; Scherer, P.E. Relation of Plasma Ceramides to Visceral Adiposity, Insulin Resistance and the Development of Type 2 Diabetes Mellitus: The Dallas Heart Study. Diabetologia 2018, 61, 2570–2579. [Google Scholar] [CrossRef] [Green Version]
- Wigger, L.; Cruciani-Guglielmacci, C.; Nicolas, A.; Denom, J.; Fernandez, N.; Fumeron, F.; Marques-Vidal, P.; Ktorza, A.; Kramer, W.; Schulte, A.; et al. Plasma Dihydroceramides Are Diabetes Susceptibility Biomarker Candidates in Mice and Humans. Cell Rep. 2017, 18, 2269–2279. [Google Scholar] [CrossRef] [Green Version]
- Hajduch, E.; Le Stunff, H. Serum Ceramides Could Predict Durable Diabetes Remission Following Gastric Bypass Surgery. Med. 2022, 3, 440–441. [Google Scholar] [CrossRef] [PubMed]
- Poss, A.M.; Krick, B.; Maschek, J.A.; Haaland, B.; Cox, J.E.; Karra, P.; Ibele, A.R.; Hunt, S.C.; Adams, T.D.; Holland, W.L.; et al. Following Roux-En-Y Gastric Bypass Surgery, Serum Ceramides Demarcate Patients That Will Fail to Achieve Normoglycemia and Diabetes Remission. Medicine 2022, 3, 452–467.e4. [Google Scholar] [CrossRef] [PubMed]
- Summers, S.A. Could Ceramides Become the New Cholesterol? Cell Metab. 2017, 27, 276–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leslie, M. Straight from the Heart. Science 2023, 379, 1080–1083. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.Y.; Holland, W.L.; Kusminski, C.M.; Sun, K.; Sharma, A.X.; Pearson, M.J.; Sifuentes, A.J.; McDonald, J.G.; Gordillo, R.; Scherer, P.E. Targeted Induction of Ceramide Degradation Leads to Improved Systemic Metabolism and Reduced Hepatic Steatosis. Cell Metab. 2015, 22, 266–278. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Ye, X.; Jiang, W.; Lu, C.; Geng, X.; Zhao, C.; Ma, Y.; Yang, P.; Man Lam, S.; Shui, G.; et al. Targeted Lipidomics Reveals Associations between Serum Sphingolipids and Insulin Sensitivity Measured by the Hyperinsulinemic-Euglycemic Clamp. Diabetes Res. Clin. Pract. 2021, 173, 108699. [Google Scholar] [CrossRef] [PubMed]
- Akhiyat, N.; Vasile, V.; Ahmad, A.; Sara, J.D.; Nardi, V.; Lerman, L.O.; Jaffe, A.; Lerman, A. Plasma Ceramide Levels Are Elevated in Patients with Early Coronary Atherosclerosis and Endothelial Dysfunction. J. Am. Heart Assoc. 2022, 11, e022852. [Google Scholar] [CrossRef] [PubMed]
- Carlier, A.; Phan, F.; Szpigel, A.; Hajduch, E.; Salem, J.-E.; Gautheron, J.; Le Goff, W.; Guérin, M.; Lachkar, F.; Ratziu, V.; et al. Dihydroceramides in Triglyceride-Enriched VLDL Are Associated with Nonalcoholic Fatty Liver Disease Severity in Type 2 Diabetes. Cell Rep. Med. 2020, 1, 100154. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, P.; Leidl, K.; Boettcher, A.; Schmitz, G.; Liebisch, G. Lipid Profiling of FPLC-Separated Lipoprotein Fractions by Electrospray Ionization Tandem Mass Spectrometry. J. Lipid Res. 2009, 50, 574–585. [Google Scholar] [CrossRef] [Green Version]
- Feingold, K.R. Lipid and Lipoprotein Metabolism. Endocrinol. Metab. Clin. North. Am. 2022, 51, 437–458. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Sakai, J.; Fujino, T.; Hattori, H.; Zenimaru, Y.; Suzuki, J.; Miyamori, I.; Yamamoto, T.T. The Very Low-Density Lipoprotein (VLDL) Receptor: Characterization and Functions as a Peripheral Lipoprotein Receptor. J. Atheroscler. Thromb. 2004, 11, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Feingold, K.R.; Anawalt, B.; Blackman, M.R.; Boyce, A.; Chrousos, G.; Corpas, E.; de Herder, W.W.; Dhatariya, K.; Dungan, K. Introduction to Lipids and Lipoproteins; In Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Bonilha, I.; Hajduch, E.; Luchiari, B.; Nadruz, W.; Le Goff, W.; Sposito, A.C. The Reciprocal Relationship between LDL Metabolism and Type 2 Diabetes Mellitus. Metabolites 2021, 11, 807. [Google Scholar] [CrossRef]
- Zelnik, I.D.; Kim, J.L.; Futerman, A.H. The Complex Tail of Circulating Sphingolipids in Atherosclerosis and Cardiovascular Disease. J. Lipid. Atheroscler. 2021, 10, 268–281. [Google Scholar] [CrossRef]
- Laaksonen, R.; Ekroos, K.; Sysi-Aho, M.; Hilvo, M.; Vihervaara, T.; Kauhanen, D.; Suoniemi, M.; Hurme, R.; März, W.; Scharnagl, H.; et al. Plasma Ceramides Predict Cardiovascular Death in Patients with Stable Coronary Artery Disease and Acute Coronary Syndromes beyond LDL-Cholesterol. Eur. Heart J. 2016, 37, 1967–1976. [Google Scholar] [CrossRef] [Green Version]
- Piccoli, M.; Cirillo, F.; Ghiroldi, A.; Rota, P.; Coviello, S.; Tarantino, A.; La Rocca, P.; Lavota, I.; Creo, P.; Signorelli, P.; et al. Sphingolipids and Atherosclerosis: The Dual Role of Ceramide and Sphingosine-1-Phosphate. Antioxidants 2023, 12, 143. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Żebrowska, E.; Chabowski, A. Insulin Resistance and Oxidative Stress in the Brain: What’s New? Int. J. Mol. Sci. 2019, 20, 874. [Google Scholar] [CrossRef] [Green Version]
- McNally, B.D.; Ashley, D.F.; Hänschke, L.; Daou, H.N.; Watt, N.T.; Murfitt, S.A.; MacCannell, A.D.V.; Whitehead, A.; Bowen, T.S.; Sanders, F.W.B.; et al. Long-Chain Ceramides Are Cell Non-Autonomous Signals Linking Lipotoxicity to Endoplasmic Reticulum Stress in Skeletal Muscle. Nat. Commun. 2022, 13, 1748. [Google Scholar] [CrossRef] [PubMed]
- Brindley, D.N.; Wang, C.N.; Mei, J.; Xu, J.; Hanna, A.N. Tumor Necrosis Factor-Alpha and Ceramides in Insulin Resistance. Lipids 1999, 34, S85–S88. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, R.; Khoury, R.; Blachnio-Zabielska, A.; Turban, S.; Loiseau, N.; Lipina, C.; Stretton, C.; Bourron, O.; Ferre, P.; Foufelle, F.; et al. Characterising the Inhibitory Actions of Ceramide upon Insulin Signaling in Different Skeletal Muscle Cell Models: A Mechanistic Insight. PLoS ONE 2014, 9, e101865. [Google Scholar] [CrossRef]
- Hajduch, E.; Balendran, A.; Batty, I.H.; Litherland, G.J.; Blair, A.S.; Downes, C.P.; Hundal, H.S. Ceramide Impairs the Insulin-Dependent Membrane Recruitment of Protein Kinase B Leading to a Loss in Downstream Signalling in L6 Skeletal Muscle Cells. Diabetologia 2001, 44, 173–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavez, J.A.; Knotts, T.A.; Wang, L.P.; Li, G.; Dobrowsky, R.T.; Florant, G.L.; Summers, S.A. A Role for Ceramide, but Not Diacylglycerol, in the Antagonism of Insulin Signal Transduction by Saturated Fatty Acids. J. Biol. Chem. 2003, 278, 10297–10303. [Google Scholar] [CrossRef] [Green Version]
- Teruel, T.; Hernandez, R.; Lorenzo, M. Ceramide Mediates Insulin Resistance by Tumor Necrosis Factor-Alpha in Brown Adipocytes by Maintaining Akt in an Inactive Dephosphorylated State. Diabetes 2001, 50, 2563–2571. [Google Scholar] [CrossRef] [Green Version]
- Summers, S.A.; Garza, L.A.; Zhou, H.; Birnbaum, M.J. Regulation of Insulin-Stimulated Glucose Transporter GLUT4 Translocation and Akt Kinase Activity by Ceramide. Mol. Cell Biol. 1998, 18, 5457–5464. [Google Scholar] [CrossRef] [Green Version]
- Ribaux, P.G.; Iynedjian, P.B. Analysis of the Role of Protein Kinase B (CAKT) in Insulin-Dependent Induction of Glucokinase and Sterol Regulatory Element-Binding Protein 1 (SREBP1) MRNAs in Hepatocytes. Biochem. J. 2003, 376, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Bandet, C.L.; Tan-Chen, S.; Ali-Berrada, S.; Campana, M.; Poirier, M.; Blachnio-Zabielska, A.; Pais-de-Barros, J.-P.; Rouch, C.; Ferré, P.; Foufelle, F.; et al. Ceramide Analog C2-Cer Induces a Loss in Insulin Sensitivity in Muscle Cells through the Salvage/Recycling Pathway. J. Biol. Chem. 2023, 299, 104815. [Google Scholar] [CrossRef]
- Boon, J.; Hoy, A.J.; Stark, R.; Brown, R.D.; Meex, R.C.; Henstridge, D.C.; Schenk, S.; Meikle, P.J.; Horowitz, J.F.; Kingwell, B.A.; et al. Ceramides Contained in LDL Are Elevated in Type 2 Diabetes and Promote Inflammation and Skeletal Muscle Insulin Resistance. Diabetes 2013, 62, 401–410. [Google Scholar] [CrossRef] [Green Version]
- Ofori, E.K.; Buabeng, A.; Amanquah, S.D.; Danquah, K.O.; Amponsah, S.K.; Dziedzorm, W.; Dogodzi, F.K.; Adusu-Donkor, L.X.; Bernard, S.K.; Asare-Anane, H. Effect of Circulating Ceramides on Adiposity and Insulin Resistance in Patients with Type 2 Diabetes: An Observational Cross-Sectional Study. Endocrinol. Diabetes Metab. 2023, 6, e418. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.N.; Fretts, A.M.; Hoofnagle, A.N.; McKnight, B.; Howard, B.V.; Umans, J.G.; Sitlani, C.M.; Siscovick, D.S.; King, I.B.; Sotoodehnia, N.; et al. Circulating Ceramides and Sphingomyelins and the Risk of Incident Cardiovascular Disease among People with Diabetes: The Strong Heart Study. Cardiovasc. Diabetol. 2022, 21, 167. [Google Scholar] [CrossRef] [PubMed]
- Fretts, A.M.; Jensen, P.N.; Hoofnagle, A.; McKnight, B.; Howard, B.V.; Umans, J.; Yu, C.; Sitlani, C.; Siscovick, D.S.; King, I.B.; et al. Plasma Ceramide Species Are Associated with Diabetes Risk in Participants of the Strong Heart Study. J. Nutr. 2020, 150, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Fretts, A.M.; Jensen, P.N.; Hoofnagle, A.N.; McKnight, B.; Howard, B.V.; Umans, J.; Sitlani, C.M.; Siscovick, D.S.; King, I.B.; Djousse, L.; et al. Plasma Ceramides Containing Saturated Fatty Acids Are Associated with Risk of Type 2 Diabetes. J. Lipid. Res. 2021, 62, 100119. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.N.; Fretts, A.M.; Yu, C.; Hoofnagle, A.N.; Umans, J.G.; Howard, B.V.; Sitlani, C.M.; Siscovick, D.S.; King, I.B.; Sotoodehnia, N.; et al. Circulating Sphingolipids, Fasting Glucose, and Impaired Fasting Glucose: The Strong Heart Family Study. EBioMedicine 2019, 41, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Watt, M.J.; Barnett, A.C.; Bruce, C.R.; Schenk, S.; Horowitz, J.F.; Hoy, A.J. Regulation of Plasma Ceramide Levels with Fatty Acid Oversupply: Evidence That the Liver Detects and Secretes de Novo Synthesised Ceramide. Diabetologia 2012, 55, 2741–2746. [Google Scholar] [CrossRef] [Green Version]
- Lightle, S.; Tosheva, R.; Lee, A.; Queen-Baker, J.; Boyanovsky, B.; Shedlofsky, S.; Nikolova-Karakashian, M. Elevation of Ceramide in Serum Lipoproteins during Acute Phase Response in Humans and Mice: Role of Serine-Palmitoyl Transferase. Arch. Biochem. Biophys 2003, 419, 120–128. [Google Scholar] [CrossRef]
- Nguyen, A.; Tao, H.; Metrione, M.; Hajri, T. Very Low Density Lipoprotein Receptor (VLDLR) Expression Is a Determinant Factor in Adipose Tissue Inflammation and Adipocyte-Macrophage Interaction. J. Biol. Chem. 2014, 289, 1688–1703. [Google Scholar] [CrossRef] [Green Version]
- Fleury, A.; Martinez, M.C.; Le Lay, S. Extracellular Vesicles as Therapeutic Tools in Cardiovascular Diseases. Front. Immunol. 2014, 5, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kita, S.; Shimomura, I. Extracellular Vesicles as an Endocrine Mechanism Connecting Distant Cells. Mol. Cells 2022, 45, 771–780. [Google Scholar] [CrossRef]
- Nojima, H.; Freeman, C.M.; Schuster, R.M.; Japtok, L.; Kleuser, B.; Edwards, M.J.; Gulbins, E.; Lentsch, A.B. Hepatocyte Exosomes Mediate Liver Repair and Regeneration via Sphingosine-1-Phosphate. J. Hepatol. 2016, 64, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Povero, D.; Eguchi, A.; Niesman, I.R.; Andronikou, N.; de Mollerat du Jeu, X.; Mulya, A.; Berk, M.; Lazic, M.; Thapaliya, S.; Parola, M.; et al. Lipid-Induced Toxicity Stimulates Hepatocytes to Release Angiogenic Microparticles That Require Vanin-1 for Uptake by Endothelial Cells. Sci. Signal 2013, 6, ra88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kornek, M.; Lynch, M.; Mehta, S.H.; Lai, M.; Exley, M.; Afdhal, N.H.; Schuppan, D. Circulating Microparticles as Disease-Specific Biomarkers of Severity of Inflammation in Patients with Hepatitis C or Nonalcoholic Steatohepatitis. Gastroenterology 2012, 143, 448–458. [Google Scholar] [CrossRef] [Green Version]
- Kakazu, E.; Mauer, A.S.; Yin, M.; Malhi, H. Hepatocytes Release Ceramide-Enriched pro-Inflammatory Extracellular Vesicles in an IRE1alpha-Dependent Manner. J. Lipid Res 2016, 57, 233–245. [Google Scholar] [CrossRef] [Green Version]
- Hirsova, P.; Ibrahim, S.H.; Krishnan, A.; Verma, V.K.; Bronk, S.F.; Werneburg, N.W.; Charlton, M.R.; Shah, V.H.; Malhi, H.; Gores, G.J. Lipid-Induced Signaling Causes Release of Inflammatory Extracellular Vesicles from Hepatocytes. Gastroenterology 2016, 150, 956–967. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, D.; Nakao, Y.; Mauer, A.S.; Thompson, J.M.; Sehrawat, T.S.; Liao, C.-Y.; Krishnan, A.; Lucien, F.; Guo, Q.; Liu, M.; et al. IRE1A Stimulates Hepatocyte-Derived Extracellular Vesicles That Promote Inflammation in Mice with Steatohepatitis. Gastroenterology 2020, 159, 1487–1503.e17. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, M.; Dasgupta, D.; Mauer, A.S.; Kakazu, E.; Nakao, K.; Malhi, H. StAR-Related Lipid Transfer Domain 11 (STARD11)-Mediated Ceramide Transport Mediates Extracellular Vesicle Biogenesis. J. Biol. Chem. 2018, 293, 15277–15289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanada, K. Intracellular Trafficking of Ceramide by Ceramide Transfer Protein. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 426–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crivelli, S.M.; Giovagnoni, C.; Zhu, Z.; Tripathi, P.; Elsherbini, A.; Quadri, Z.; Pu, J.; Zhang, L.; Ferko, B.; Berkes, D.; et al. Function of Ceramide Transfer Protein for Biogenesis and Sphingolipid Composition of Extracellular Vesicles. J. Extracell Vesicles 2022, 11, e12233. [Google Scholar] [CrossRef]
- Bandet, C.L.; Mahfouz, R.; Veret, J.; Sotiropoulos, A.; Poirier, M.; Giussani, P.; Campana, M.; Philippe, E.; Blachnio-Zabielska, A.; Ballaire, R.; et al. Ceramide Transporter CERT Is Involved in Muscle Insulin Signaling Defects Under Lipotoxic Conditions. Diabetes 2018, 67, 1258–1271. [Google Scholar] [CrossRef] [Green Version]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Straczkowski, M.; Kowalska, I.; Nikolajuk, A.; Dzienis-Straczkowska, S.; Kinalska, I.; Baranowski, M.; Zendzian-Piotrowska, M.; Brzezinska, Z.; Gorski, J. Relationship between Insulin Sensitivity and Sphingomyelin Signaling Pathway in Human Skeletal Muscle. Diabetes 2004, 53, 1215–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sindhu, S.; Leung, Y.H.; Arefanian, H.; Madiraju, S.R.M.; Al-Mulla, F.; Ahmad, R.; Prentki, M. Neutral Sphingomyelinase-2 and Cardiometabolic Diseases. Obes. Rev. 2021, 22, e13248. [Google Scholar] [CrossRef]
- Aswad, H.; Forterre, A.; Wiklander, O.P.B.; Vial, G.; Danty-Berger, E.; Jalabert, A.; Lamazière, A.; Meugnier, E.; Pesenti, S.; Ott, C.; et al. Exosomes Participate in the Alteration of Muscle Homeostasis during Lipid-Induced Insulin Resistance in Mice. Diabetologia 2014, 57, 2155–2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentino, T.R.; Rule, B.D.; Mobley, C.B.; Nikolova-Karakashian, M.; Vechetti, I.J. Skeletal Muscle Cell Growth Alters the Lipid Composition of Extracellular Vesicles. Membranes 2021, 11, 619. [Google Scholar] [CrossRef]
- Mebarek, S.; Komati, H.; Naro, F.; Zeiller, C.; Alvisi, M.; Lagarde, M.; Prigent, A.F.; Nemoz, G. Inhibition of de Novo Ceramide Synthesis Upregulates Phospholipase D and Enhances Myogenic Differentiation. J. Cell Sci. 2007, 120, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Michel, L.Y.M. Extracellular Vesicles in Adipose Tissue Communication with the Healthy and Pathological Heart. Int. J. Mol. Sci. 2023, 24, 7745. [Google Scholar] [CrossRef]
- Blandin, A.; Dugail, I.; Hilairet, G.; Ponnaiah, M.; Ghesquière, V.; Froger, J.; Ducheix, S.; Fizanne, L.; Boursier, J.; Cariou, B.; et al. Lipidomic Analysis of Adipose-Derived Extracellular Vesicles Reveals Specific EV Lipid Sorting Informative of the Obesity Metabolic State. Cell Rep. 2023, 42, 112169. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.D.; Morelli, A.E. Regulation of Immune Responses by Extracellular Vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.K.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome Secreted by MSC Reduces Myocardial Ischemia/Reperfusion Injury. Stem. Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Denzel, M.S.; Scimia, M.-C.; Zumstein, P.M.; Walsh, K.; Ruiz-Lozano, P.; Ranscht, B. T-Cadherin Is Critical for Adiponectin-Mediated Cardioprotection in Mice. J. Clin. Investig. 2010, 120, 4342–4352. [Google Scholar] [CrossRef] [Green Version]
- Obata, Y.; Kita, S.; Koyama, Y.; Fukuda, S.; Takeda, H.; Takahashi, M.; Fujishima, Y.; Nagao, H.; Masuda, S.; Tanaka, Y.; et al. Adiponectin/T-Cadherin System Enhances Exosome Biogenesis and Decreases Cellular Ceramides by Exosomal Release. JCI Insight 2018, 3, e99680. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Martinez, I.; Alen, R.; Pereira, L.; Povo-Retana, A.; Astudillo, A.M.; Hitos, A.B.; Gomez-Hurtado, I.; Lopez-Collazo, E.; Boscá, L.; Francés, R.; et al. Saturated Fatty Acid-Enriched Small Extracellular Vesicles Mediate a Crosstalk Inducing Liver Inflammation and Hepatocyte Insulin Resistance. JHEP Rep. 2023, 5, 100756. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, I.; Alen, R.; Rada, P.; Valverde, A.M. Insights into Extracellular Vesicles as Biomarker of NAFLD Pathogenesis. Front. Med. 2020, 7, 395. [Google Scholar] [CrossRef] [PubMed]
- Kurzawa-Akanbi, M.; Tammireddy, S.; Fabrik, I.; Gliaudelytė, L.; Doherty, M.K.; Heap, R.; Matečko-Burmann, I.; Burmann, B.M.; Trost, M.; Lucocq, J.M.; et al. Altered Ceramide Metabolism Is a Feature in the Extracellular Vesicle-Mediated Spread of Alpha-Synuclein in Lewy Body Disorders. Acta Neuropathol. 2021, 142, 961–984. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Lee, P.-T.; Chen, K.; Mao, D.; Tan, K.L.; Zuo, Z.; Lin, W.-W.; Wang, L.; Bellen, H.J. Phospholipase PLA2G6, a Parkinsonism-Associated Gene, Affects Vps26 and Vps35, Retromer Function, and Ceramide Levels, Similar to α-Synuclein Gain. Cell Metab. 2018, 28, 605–618.e6. [Google Scholar] [CrossRef] [Green Version]
- Nigro, J.; Osman, N.; Dart, A.M.; Little, P.J. Insulin Resistance and Atherosclerosis. Endocr. Rev. 2006, 27, 242–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rütti, S.; Ehses, J.A.; Sibler, R.A.; Prazak, R.; Rohrer, L.; Georgopoulos, S.; Meier, D.T.; Niclauss, N.; Berney, T.; Donath, M.Y.; et al. Low- and High-Density Lipoproteins Modulate Function, Apoptosis, and Proliferation of Primary Human and Murine Pancreatic Beta-Cells. Endocrinology 2009, 150, 4521–4530. [Google Scholar] [CrossRef] [Green Version]
- Roehrich, M.-E.; Mooser, V.; Lenain, V.; Herz, J.; Nimpf, J.; Azhar, S.; Bideau, M.; Capponi, A.; Nicod, P.; Haefliger, J.-A.; et al. Insulin-Secreting Beta-Cell Dysfunction Induced by Human Lipoproteins. J. Biol. Chem. 2003, 278, 18368–18375. [Google Scholar] [CrossRef] [Green Version]
- Yalcinkaya, M.; Kerksiek, A.; Gebert, K.; Annema, W.; Sibler, R.; Radosavljevic, S.; Lütjohann, D.; Rohrer, L.; von Eckardstein, A. HDL Inhibits Endoplasmic Reticulum Stress-Induced Apoptosis of Pancreatic β-Cells in Vitro by Activation of Smoothened. J. Lipid Res. 2020, 61, 492–504. [Google Scholar] [CrossRef] [Green Version]
- Christoffersen, C.; Obinata, H.; Kumaraswamy, S.B.; Galvani, S.; Ahnstrom, J.; Sevvana, M.; Egerer-Sieber, C.; Muller, Y.A.; Hla, T.; Nielsen, L.B.; et al. Endothelium-Protective Sphingosine-1-Phosphate Provided by HDL-Associated Apolipoprotein M. Proc. Natl. Acad. Sci. USA 2011, 108, 9613–9618. [Google Scholar] [CrossRef]
- Bisgaard, L.S.; Christoffersen, C. Apolipoprotein M/Sphingosine-1-Phosphate: Novel Effects on Lipids, Inflammation and Kidney Biology. Curr. Opin. Lipidol. 2019, 30, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Cervin, C.; Axler, O.; Holmkvist, J.; Almgren, P.; Rantala, E.; Tuomi, T.; Groop, L.; Dahlbäck, B.; Karlsson, E. An Investigation of Serum Concentration of ApoM as a Potential MODY3 Marker Using a Novel ELISA. J. Intern. Med. 2010, 267, 316–321. [Google Scholar] [CrossRef]
- Kurano, M.; Tsukamoto, K.; Shimizu, T.; Kassai, H.; Nakao, K.; Aiba, A.; Hara, M.; Yatomi, Y. Protection Against Insulin Resistance by Apolipoprotein M/Sphingosine-1-Phosphate. Diabetes 2020, 69, 867–881. [Google Scholar] [CrossRef] [PubMed]
- Kurano, M.; Hara, M.; Tsuneyama, K.; Sakoda, H.; Shimizu, T.; Tsukamoto, K.; Ikeda, H.; Yatomi, Y. Induction of Insulin Secretion by Apolipoprotein M, a Carrier for Sphingosine 1-Phosphate. Biochim. Biophys. Acta 2014, 1841, 1217–1226. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zhang, J.; Yao, S.; Pan, L.; Luo, G.; Xu, N. Apolipoprotein M Overexpression through Adeno-Associated Virus Gene Transfer Improves Insulin Secretion and Insulin Sensitivity in Goto-Kakizaki Rats. J. Diabetes Investig. 2020, 11, 1150–1158. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, M.C.; Shanmugarajah, N.; Lee, S.X.; Kraakman, M.J.; Westerterp, M.; Kitamoto, T.; Harris, M.; Cook, J.R.; Gusarova, G.A.; Zhong, K.; et al. Hepatic FoxOs Link Insulin Signaling with Plasma Lipoprotein Metabolism through an Apolipoprotein M/Sphingosine-1-Phosphate Pathway. J. Clin. Investig. 2022, 132, e146219. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kurano, M.; Nanya, M.; Shimizu, T.; Ohkawa, R.; Tozuka, M.; Yatomi, Y. Glycation of HDL Polymerizes Apolipoprotein M and Attenuates Its Capacity to Bind to Sphingosine 1-Phosphate. J. Atheroscler. Thromb. 2021, 28, 730–741. [Google Scholar] [CrossRef]
- Christoffersen, C.; Federspiel, C.K.; Borup, A.; Christensen, P.M.; Madsen, A.N.; Heine, M.; Nielsen, C.H.; Kjaer, A.; Holst, B.; Heeren, J.; et al. The Apolipoprotein M/S1P Axis Controls Triglyceride Metabolism and Brown Fat Activity. Cell Rep. 2018, 22, 175–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Therond, P.; Chapman, M.J. Sphingosine-1-Phosphate: Metabolism, Transport, Atheroprotection and Effect of Statin Treatment. Curr. Opin. Lipidol. 2022, 33, 199–207. [Google Scholar] [CrossRef]
- Liao, C.-Y.; Song, M.J.; Gao, Y.; Mauer, A.S.; Revzin, A.; Malhi, H. Hepatocyte-Derived Lipotoxic Extracellular Vesicle Sphingosine 1-Phosphate Induces Macrophage Chemotaxis. Front. Immunol. 2018, 9, 2980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verderio, C.; Gabrielli, M.; Giussani, P. Role of Sphingolipids in the Biogenesis and Biological Activity of Extracellular Vesicles. J. Lipid Res. 2018, 59, 1325–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, J.; Walsh, M.T.; Hammad, S.M.; Cuchel, M.; Tarugi, P.; Hegele, R.A.; Davidson, N.O.; Rader, D.J.; Klein, R.L.; Hussain, M.M. Microsomal Triglyceride Transfer Protein Transfers and Determines Plasma Concentrations of Ceramide and Sphingomyelin but Not Glycosylceramide. J. Biol. Chem. 2015, 290, 25863–25875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, C.; Mullan, K.; Owens, D.; Tomkin, G.H. Intestinal Microsomal Triglyceride Transfer Protein in Type 2 Diabetic and Non-Diabetic Subjects: The Relationship to Triglyceride-Rich Postprandial Lipoprotein Composition. Atherosclerosis 2006, 187, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Sponton, C.H.; Hosono, T.; Taura, J.; Jedrychowski, M.P.; Yoneshiro, T.; Wang, Q.; Takahashi, M.; Matsui, Y.; Ikeda, K.; Oguri, Y.; et al. The Regulation of Glucose and Lipid Homeostasis via PLTP as a Mediator of BAT-Liver Communication. EMBO Rep. 2020, 21, e49828. [Google Scholar] [CrossRef] [PubMed]
- Horibe, S.; Tanahashi, T.; Kawauchi, S.; Murakami, Y.; Rikitake, Y. Mechanism of Recipient Cell-Dependent Differences in Exosome Uptake. BMC Cancer 2018, 18, 47. [Google Scholar] [CrossRef] [Green Version]
- Young, M.M.; Wang, H.-G. Sphingolipids as Regulators of Autophagy and Endocytic Trafficking. Adv. Cancer Res. 2018, 140, 27–60. [Google Scholar] [CrossRef]
- Latteri, S.; Sofia, M.; Puleo, S.; Di Vincenzo, A.; Cinti, S.; Castorina, S. Mechanisms Linking Bariatric Surgery to Adipose Tissue, Glucose Metabolism, Fatty Liver Disease and Gut Microbiota. Langenbecks Arch. Surg. 2023, 408, 101. [Google Scholar] [CrossRef] [PubMed]
- Kayser, B.D.; Prifti, E.; Lhomme, M.; Belda, E.; Dao, M.-C.; Aron-Wisnewsky, J.; MICRO-Obes Consortium; Kontush, A.; Zucker, J.-D.; Rizkalla, S.W.; et al. Elevated Serum Ceramides Are Linked with Obesity-Associated Gut Dysbiosis and Impaired Glucose Metabolism. Metabolomics 2019, 15, 140. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali-Berrada, S.; Guitton, J.; Tan-Chen, S.; Gyulkhandanyan, A.; Hajduch, E.; Le Stunff, H. Circulating Sphingolipids and Glucose Homeostasis: An Update. Int. J. Mol. Sci. 2023, 24, 12720. https://doi.org/10.3390/ijms241612720
Ali-Berrada S, Guitton J, Tan-Chen S, Gyulkhandanyan A, Hajduch E, Le Stunff H. Circulating Sphingolipids and Glucose Homeostasis: An Update. International Journal of Molecular Sciences. 2023; 24(16):12720. https://doi.org/10.3390/ijms241612720
Chicago/Turabian StyleAli-Berrada, Sarah, Jeanne Guitton, Sophie Tan-Chen, Anna Gyulkhandanyan, Eric Hajduch, and Hervé Le Stunff. 2023. "Circulating Sphingolipids and Glucose Homeostasis: An Update" International Journal of Molecular Sciences 24, no. 16: 12720. https://doi.org/10.3390/ijms241612720