Highly Diluted Glyphosate Mitigates Its Effects on Artemia salina: Physicochemical Implications
Abstract
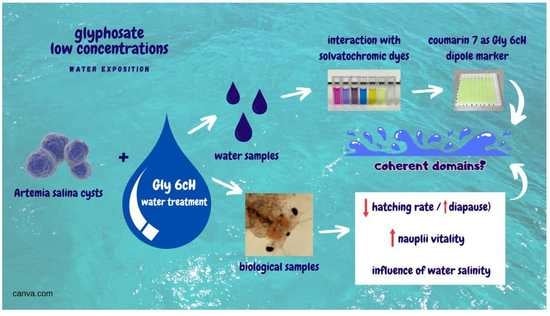
:1. Introduction
Objective
2. Results
2.1. Analysis of the Nauplii Behavior and Vitality
2.2. Analysis of Nauplii Morphology
2.3. Analysis of the Hatching Rate
2.4. Analysis of Hatching According to the Treatment and Salinity
2.5. Analysis of Hatching According to the Intensity of the Challenge
2.6. Analysis of Nauplii Activity
2.7. Physicochemical Analysis with Solvatochromic Dyes
2.7.1. Experiment 1
2.7.2. Experiment 2
3. Discussion
4. Materials and Methods
4.1. Ethics
4.2. Potentized Glyphosate Preparation
4.3. Experimental Design
4.3.1. Experiment 1
4.3.2. Experiment 2
4.4. Physicochemical Analysis of Water
4.4.1. Sample Preparation for Analysis and Coding
4.4.2. Spectrophotometry with Solvatochromic Dyes
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zanella, T.V. Poluição Marinha por Plásticos e o Direito Internacional do Ambiente. Lisboa, RIDB. 2013. Available online: https://www.cidp.pt/revistas/ridb/2013/12/2013_12_14473_14500.pdf (accessed on 15 November 2019).
- Albendín, M.G.; Aranda, V.; Coello, M.D.; González-Gómez, C.; Rodríguez-Barroso, R.; Quiroga, J.M.; Arellano, J.M. Pharmaceutical Products and Pesticides Toxicity Associated with Microplastics (Polyvinyl Chloride) in Artemia salina. Int. J. Environ. Res. Public Health 2021, 18, 10773. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, R.; Scalisi, E.M.; Messina, G.; Fragalà, G.; Ignoto, S.; Salvaggio, A.; Zimbone, M.; Impellizzeri, G.; Brundo, M.V. Artemia salina: A microcrustacean to assess engineered nanoparticles toxicity. Microsc. Res. Technol. 2021, 84, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Lavens, P.; Sorgeloos, P. Manual on the Production and Use of Live Food for Aquaculture. FAO Fisheries Technical Paper. 2006. Available online: https://www.researchgate.net/publication/285237285 (accessed on 10 January 2021).
- Kalčíková, G.; Zagorc-Končan, J.; Gotvajn, A. Artemia salina acute immobilization test: A possible tool for aquatic ecotoxicity assessment. Water Sci. Technol. 2012, 66, 903–908. [Google Scholar] [CrossRef]
- Bustos-Obregon, E.; Vargas, A. Chronic toxicity bioassay with populations of the crustacean Artemia salina exposed to the or-ganophosphate diazinon. Biol. Res. 2010, 43, 357–362. [Google Scholar] [CrossRef]
- Chen, B.; Chu, T.-W.; Chiu, K.; Hong, M.-C.; Wu, T.-M.; Ma, J.-W.; Liang, C.-M.; Wang, W.-K. Transcriptomic analysis elucidates the molecular processes associated with hydrogen peroxide-induced diapause termination in Artemia-encysted embryos. PLoS ONE 2021, 16, e0247160. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.A.G.; Nagai, M.Y.d.O.; Coimbra, E.N.; Mohammad, S.N.; Silva, J.S.; Von Ancken, A.; Pinto, S.A.G.; Aguiar, M.S.; Dutra-Correa, M.; Hortellani, M.A.; et al. Bioresilience to Mercury Chloride of the Brine Shrimp Artemia Salina after Treatment with Homeopathic Mercurius Corrosivus. Homeopathy 2021, 110, 244–255. [Google Scholar] [CrossRef]
- Mohammad, S.N.; Pinto, A.A.G.; Nagai, M.Y.O.; Coimbra, E.M.; Suffredini, I.B.; Peres, G.B.; Bernardi, M.M.; Bonamin, L.V. Interference of Water and Environmental Variables on Lead Chloride Toxicity in Artemia salina Model. Water J. 2022. Special Edition for the 9th World Water Forum Dakar, Senegal, March 2022. [Google Scholar] [CrossRef]
- Muñoz, J.P.; Bleak, T.C.; Calaf, G.M. Glyphosate and the key characteristics of an endocrine disruptor: A review. Chemosphere 2021, 270, 128619. [Google Scholar] [CrossRef]
- Foldager, L.; Winters, J.F.M.; Nørskov, N.P.; Sørensen, M.T. Impact of feed glyphosate residues on broiler breeder egg production and egg hatchability. Sci. Rep. 2021, 11, 19290. [Google Scholar] [CrossRef]
- Mercurio, P.; Flores, F.; Mueller, J.F.; Carter, S.; Negri, A.P. Glyphosate persistence in seawater. Mar. Pollut. Bull. 2014, 85, 385–390. [Google Scholar] [CrossRef]
- Mercurio, P.; Mueller, J.F.; Eaglesham, G.; Flores, F.; Negri, A.P. Herbicide Persistence in Seawater Simulation Experiments. PLoS ONE 2015, 10, e0136391. [Google Scholar] [CrossRef]
- Slaby, S.; Titran, P.; Marchand, G.; Hanotel, J.; Lescuyer, A.; Leprêtre, A.; Bodart, J.F.; Marin, M.; Lemiere, S. Effects of glyphosate and a commercial formulation Roundup® exposures on the maturation of Xenopus laevis oocytes. Environ. Sci. Pollut. Res. Int. 2020, 27, 3697–3705. [Google Scholar] [CrossRef]
- Ren, X.; Dai, P.; Perveen, A.; Tang, Q.; Zhao, L.; Jia, X.; Li, Y.; Li, C. Effects of chronic glyphosate exposure to pregnant mice on hepatic lipid metabolism in offspring. Environ. Pollut. 2019, 254 Pt A, 112906. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Proposal for a Regulation of the European Parliament and of the Council on the Sustainable Use of Plant Protection Products and Amending Regulation (EU) 2021/2115. 71p. 2022. Available online: https://food.ec.europa.eu/system/files/2022-06/pesticides_sud_eval_2022_reg_2022-305_en.pdf (accessed on 8 May 2023).
- Nagai, M.Y.O.; Von Ancken, A.C.B.; Bonamin, L.V. Effects of Highly Diluted Substances on Aquatic Animals: A Review. Water J. 2022. Special Edition for the 9th World Water Forum Dakar, Senegal, March 2022. [Google Scholar] [CrossRef]
- Segat, H.J.; Metz, V.G.; Rosa, H.Z.; Dias, V.T.; Barcelos, R.C.; Dolci, G.S.; Burger, M.E. Substitution therapy with amphetamine-isotherapic attenuates amphetamine toxicological aspects of addiction. Neurosci. Lett. 2019, 690, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Bagot, J.-L.; Information, P.E.K.F.C. Using hetero-isotherapics in cancer supportive care: The fruit of fifteen years of experience. Homeopathy 2016, 105, 119–125. [Google Scholar] [CrossRef]
- Orjales, I.; López-Alonso, M.; Rodríguez-Bermúdez, R.; Rey-Crespo, F.; Villar, A.; Miranda, M. Use of homeopathy in organic dairy farming in Spain. Homeopathy 2016, 105, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.M.; Mangeiro, M.Z.; Almeida, A.M.; Almeida, R.N.; Souza, R.M. Effect of Nosodes on Lettuce, Parasitized or Not by Meloidogyne enterolobii. Homeopathy 2021, 110, 256–262. [Google Scholar] [CrossRef]
- Betti, L.; Trebbi, G.; Majewsky, V.; Scherr, C.; Shah-Rossi, D.; Jäger, T.; Baumgartner, S. Use of homeopathic preparations in phytopathological models and in field trials: A critical review. Homeopathy 2009, 98, 244–266. [Google Scholar] [CrossRef]
- Aparicio, A.C.C.; de Oliveira, L.H.S.; Silva, J.S.; Coelho, C.P.; Pinheiro, S.R.; Souza, M.F.; Suffredini, I.B.; Cartwright, S.J.; Bonamin, L.V. Interaction between Solvatochromic Dyes and Water Sampled from a Natural Source Treated with High Dilutions of Phosphorus. Homeopathy 2020, 109, 126–132. [Google Scholar] [CrossRef]
- Yinnon, T.A.; Yinnon, C.A. Electric dipole aggregates in very dilute polar liquids: Theory and experimental evidence. Int. J. Mod. Phys. B 2011, 25, 3707–3743. [Google Scholar] [CrossRef]
- Macrae, T.H. Stress tolerance during diapause and quiescence of the brine shrimp, Artemia. Cell Stress Chaperon 2016, 21, 9–18. [Google Scholar] [CrossRef] [PubMed]
- King, A.M.; Toxopeus, J.; MacRae, T.H. Functional differentiation of small heat shock proteins in diapause-destined Artemia embryos. FEBS J. 2013, 280, 4761–4772. [Google Scholar] [CrossRef] [PubMed]
- Demangeat, J.-L. NMR relaxation evidence for solute-induced nanosized superstructures in ultramolecular aqueous dilutions of silica–lactose. J. Mol. Liq. 2010, 155, 71–79. [Google Scholar] [CrossRef]
- Shahabi, S.; Borneman, J.P. The Electrostatic Model of Homeopathy: The Mechanism of Physicochemical Activities of Homeopathic Medicines. Homeopathy 2022, 111, 210–216. [Google Scholar] [CrossRef]
- Smedbol; Gomes, M.P.; Paquet, S.; Labrecque, M.; Lepage, L.; Lucotte, M.; Juneau, P. Effects of low concentrations of glyphosate-based herbicide factor 540® on an agricultural stream freshwater phytoplankton community. Chemosphere 2018, 192, 133–141. [Google Scholar] [CrossRef]
- Brovini, E.M.; Cardoso, S.J.; Quadra, G.R.; Vilas-Boas, J.A.; Paranaíba, J.R.; Pereira, R.O.; Mendonça, R.F. Glyphosate concentrations in global freshwaters: Are aquatic organisms at risk? Environ. Sci. Pollut. Res. Int. 2021, 28, 60635–60648. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Gill, J.P.K.; Datta, S.; Singh, S.; Dhaka, V.; Kapoor, D.; Wani, A.B.; Dhanjal, D.S.; Kumar, M.; et al. Herbicide Glyphosate: Toxicity and Microbial Degradation. Int. J. Environ. Res. Public Health 2020, 17, 7519. [Google Scholar] [CrossRef]
- López-Carvallo, J.A.; Mazón-Suástegui, J.M.; Hernández-Oñate, M.; Ramírez, D.T.; Abasolo-Pacheco, F.; Morelos-Castro, R.M.; Arcos-Ortega, G.F. Transcriptome analysis of Catarina scallop (Argopecten ventricosus) juveniles treated with highly-diluted immunomodulatory compounds reveals activation of non-self-recognition system. PLoS ONE 2020, 15, e0233064. [Google Scholar] [CrossRef]
- Manzalini, A.; Galeazzi, B. Explaining Homeopathy with Quantum Electrodynamics. Homeopathy 2019, 32, 169–176. [Google Scholar] [CrossRef]
- Elia, V.; Napoli, E. Dissipative structures in extremely diluted solutions of homeopathic medicines: A molecular model based on physico-chemical and gravimetric evidences. Int. J. Des. Nat. Ecodyn. 2010, 5, 39–48. [Google Scholar] [CrossRef]
- Klein, S.; Würtenberger, S.; Wolf, U.; Baumgartner, S.; Tournier, A. Physicochemical Investigations of Homeopathic Preparations: A Systematic Review and Bibliometric Analysis—Part 1. J. Altern. Complement. Med. 2018, 24, 409–421. [Google Scholar] [CrossRef]
- Del Giudice, E.; Preparata, G.; Vitiello, G. Water as a Free Electric Dipole Laser. Phys. Rev. Lett. 1988, 61, 1085–1088. [Google Scholar] [CrossRef]
- Messori, C.; Prinzera, S.V.; di Bardone, F.B. Deep into the water: Exploring the hydro-electromagnetic and quantum-electrodynamic properties of interfacial water in living systems. Open Access Libr. J. 2019, 6, 1. [Google Scholar] [CrossRef]
- Scirè, A. A mesoscopic model for the collective dynamics of water coherence domains. arXiv 2020, arXiv:2004.07545. [Google Scholar] [CrossRef]
- Yinnon, T.A. Very Dilute Aqueous Solutions—Structural and Electromagnetic Phenomena. Water J. 2017, 9, 28–66. [Google Scholar] [CrossRef]
- Yinnon, T.A. Liquids Prepared by Serially Diluting and Vigorously Shaking of Aqueous Solutions: Unveiling Effects of the Solute on their Properties. Water J. 2020, 10, 115–134. [Google Scholar] [CrossRef]
- Del Giudice, E.; Preparata, G.; Fleischmann, M. QED coherence and electrolyte solutions. J. Electroanal. Chem. 2000, 482, 110–116. [Google Scholar] [CrossRef]
- Yinnon, T.A.; Liu, Z.-Q. Domains Formation Mediated by Electromagnetic Fields in Very Dilute Aqueous Solutions: 3. Quantum Electrodynamic Analyses of Experimental Data on Solutions of Weak Electrolytes and Non-electrolytes. Water J. 2015, 7, 70–95. [Google Scholar]
- Mahata, C. Dielectric dispersion studies of some potentised homeopathic medicines reveal structured vehicle. Homeopathy 2013, 102, 262–267. [Google Scholar] [CrossRef]
- Arani, R.; Bono, I.; Del Giudice, E.; Preparata, G. QED coherence and the thermodynamics of water. Int. J. Mod. Phys. B 1995, 9, 1813–1841. [Google Scholar] [CrossRef]
- Bono, I.; Del Giudice, E.; Gamberale, L.; Henry, M. Emergence of the Coherent Structure of Liquid Water. Water 2012, 4, 510–532. [Google Scholar] [CrossRef]
- Bonamin, L.V.; Pedro, R.R.P.; Mota, H.M.G.; Aguiar, M.S.C.; Pinto, S.A.G.; de Souza, J.; de Oliveira, L.H.S.; Aparicio, A.C.; Peres, G.B.; Suffredini, I.; et al. Characterization of Antimonium crudum Activity Using Solvatochromic Dyes. Homeopathy 2019, 109, 79–86. [Google Scholar] [CrossRef]
- Cartwright, S.J. Homeopathic Potencies May Possess an Electric Field(-like) Component: Evidence from the Use of Encapsulated Solvatochromic Dyes. Homeopathy 2020, 109, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, S.J. Solvatochromic dyes detect the presence of homeopathic potencies. Homeopathy 2016, 105, 55–65. [Google Scholar] [CrossRef]
- Cartwright, S.J. Interaction of homeopathic potencies with the water soluble solvatochromic dye bis-dimethylaminofuchsone. Part 1: pH studies. Homeopathy 2017, 106, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, S.J. Degree of Response to Homeopathic Potencies Correlates with Dipole Moment Size in Molecular Detectors: Implications for Understanding the Fundamental Nature of Serially Diluted and Succussed Solutions. Homeopathy 2018, 107, 19–31. [Google Scholar] [CrossRef]
- Cartwright, S.J. Immobilization of Solvatochromic Dyes on Transparent Cellulose Films: An Improved Method for the Study of Homeopathic Potencies. Homeopathy 2022, 112, 125–134. [Google Scholar] [CrossRef]
- Ghilosso-Bortolini, R.; Bonamin, L.; Holandino, C. Putative protective effect of Cadmium chloride high diluted solution on LLC-PK1 cell intoxicated by high concentration of this same metal: An isopathic in vitro assay. Int. J. High Dilution Res. 2010, 9, 16–29. [Google Scholar] [CrossRef]
- Banerjee, P.; Bhattacharyya, S.S.; Pathak, S.; Boujedaini, N.; Belon, P.; Khuda-Bukhsh, A.R. Evidence of protective potentials of micro-doses of ultra-high diluted arsenic trioxide in mice receiving repeated injections of arsenic trioxide. Evid Based Complement. Alternat. Med. 2011, 2011, 391752. [Google Scholar] [CrossRef]
- Das, D.; De, A.; Dutta, S.; Biswas, R.; Boujedaini, N.; Khuda-Bukhsh, A.R. Potentized homeopathic drug Arsenicum album 30C positively modulates protein biomarkers and gene expressions in Saccharomyces cerevisiae exposed to arsenate. J. Chin. Integr. Med. 2011, 9, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Biswas, S.J.; Khuda-Bukhsh, A.R. Comparative Efficacy of Pre-feeding, Post-feeding and Combined Pre- and Post-feeding of Two Microdoses of a Potentized Homeopathic Drug, Mercurius Solubilis, in Ameliorating Genotoxic Effects Produced by Mercuric Chloride in Mice. Evid.-Based Complement. Altern. Med. 2004, 1, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Lahnstein, L.; Binder, M.; Thurneysen, A.; Frei-Erb, M.; Betti, L.; Peruzzi, M.; Heusser, P.; Baumgartner, S. Isopathic treatment effects of Arsenicum album 45x on wheat seedling growth—Further reproduction trials. Homeopathy 2009, 98, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Brown, J.J. Influence of fluctuating salinity on insecticide tolerance of two euryhaline arthropods. J. Econ. Entomol. 2006, 99, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.G.; Kültz, D. The cellular stress response in fish exposed to salinity fluctuations. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2020, 333, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Elwood, R. Pain and suffering in invertebrates? ILAR J. 2011, 52, 175–184. [Google Scholar] [CrossRef]
- BRASIL. Brazilian Homeopathic Pharmacopoeia—ANVISA. Available online: http://antigo.anvisa.gov.br/documents/33832/259147/Farmacopeia+HOMEOPATICA+3a+EDICAO+INGLES+com+alerta.pdf/49a48a50-0d3e-4ab9-bc41-eb361c8afbb1 (accessed on 8 November 2022).
- Endler, P.C.; Pongratz, W.; Smith, C.W.; Schulte, J. Non-molecular information transfer from thyroxine to frogs with regard to homeopathic toxicology. Vet. Hum. Toxicol. 1995, 37, 259–260. [Google Scholar]
- Kanissery, R.; Gairhe, B.; Kadyampakeni, D.; Batuman, O.; Alferez, F. Glyphosate: Its Environmental Persistence and Impact on Crop Health and Nutrition. Plants 2019, 8, 499. [Google Scholar] [CrossRef]
- Marques, J.G.D.C.; Veríssimo, K.J.D.S.; Fernandes, B.S.; Ferreira, S.R.D.M.; Montenegro, S.M.G.L.; Motteran, F. Glyphosate: A Review on the Current Environmental Impacts from a Brazilian Perspective. Bull. Environ. Contam. Toxicol. 2021, 107, 385–397. [Google Scholar] [CrossRef]
- Phansalkar, N.; More, S.; Sabale, A.; Joshi, M. Adaptive local thresholding for detection of nuclei in diversity stained cytology images. In Proceedings of the 2011 International Conference on Communications and Signal Processing, Calicut, India, 10–12 February 2011; pp. 218–220. [Google Scholar]











| Plate | Plate 1 (Glyphosate CL 10) | Plate 2 (Glyphosate CL30) | Plate 3 (Glyphosate CL50) | Plate 4 (Unchallenged Cysts) | |
|---|---|---|---|---|---|
| Salinity | |||||
| 180 µL of 90% seawater | 20 µL Gly 6 cH | 0.02% | 0.03% | 0.05% | zero |
| 180 µL of 80% seawater | 20 µL Gly 6 cH | 0.02% | 0.03% | 0.05% | zero |
| 180 µL of 60% seawater | 20 µL Gly 6 cH | 0.02% | 0.03% | 0.05% | zero |
| 180 µL of 50% seawater | 20 µL Gly 6 cH | 0.02% | 0.03% | 0.05% | zero |
| 180 µL of 100% seawater | 20 µL pure water | 0.02% | 0.03% | 0.05% | zero |
| 180 µL of 100% seawater | 20 µL succussed pure water | 0.02% | 0.03% | 0.05% | zero |
| 200 µL of 100% seawater (baseline) | - | 0.02% | 0.03% | 0.05% | zero |
| Dye | CAS | Color in Alcohol | Molarity | Wavelength |
|---|---|---|---|---|
| REICHARDT’S DYE (ET30) | 10081-39-7 | violet | 200 µM | 550 nm |
| COUMARIN 7 | 27425-55-4 | fluorescent green/yellow | 25 µM | 433 nm |
| RHODANINE [5—(4–DIMETYLAMINEBENZILIDENE)] | 536-17-4 | yellow | 50 µM | 457 nm |
| ET 33 (-) 2,6-DICHLOR-4-(TRIPHENILPIRIDINIUM-1-IL)-PHENOLATE | 121792-58-3 | orange | 245 µM | 484 nm |
| NILE RED | 7385-67-3 | pink | 20 µM | 550 nm |
| METHYLENE VIOLET | 2516-05-4 | purple | 50 µM | 600 nm |
| N, NDIMETHYLINDOANILINE | 2150-58-5 | blue | 25 µM | 595 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, M.Y.D.d.O.; Mohammad, S.N.; Pinto, A.A.G.; Coimbra, E.N.; Peres, G.B.; Suffredini, I.B.; Bernardi, M.M.; Tournier, A.L.; Jerman, I.; Cartwright, S.J.; et al. Highly Diluted Glyphosate Mitigates Its Effects on Artemia salina: Physicochemical Implications. Int. J. Mol. Sci. 2023, 24, 9478. https://doi.org/10.3390/ijms24119478
Nagai MYDdO, Mohammad SN, Pinto AAG, Coimbra EN, Peres GB, Suffredini IB, Bernardi MM, Tournier AL, Jerman I, Cartwright SJ, et al. Highly Diluted Glyphosate Mitigates Its Effects on Artemia salina: Physicochemical Implications. International Journal of Molecular Sciences. 2023; 24(11):9478. https://doi.org/10.3390/ijms24119478
Chicago/Turabian StyleNagai, Mirian Yaeko Dias de Oliveira, Suham Nowrooz Mohammad, Andreia Adelaide G. Pinto, Ednar Nascimento Coimbra, Giovani Bravin Peres, Ivana Barbosa Suffredini, Maria Martha Bernardi, Alexander L. Tournier, Igor Jerman, Steven John Cartwright, and et al. 2023. "Highly Diluted Glyphosate Mitigates Its Effects on Artemia salina: Physicochemical Implications" International Journal of Molecular Sciences 24, no. 11: 9478. https://doi.org/10.3390/ijms24119478