Four-Dimensional Printing and Shape Memory Materials in Bone Tissue Engineering
Abstract
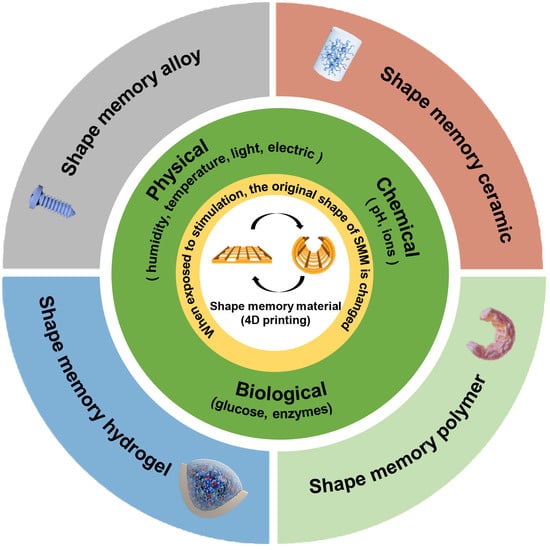
:1. Introduction
2. The Mechanism and Design Principle of 4D Printing
3. Research Progress of Bone Histology in Various Categories of 3D Printing
3.1. DIW
3.2. FDM
3.3. SLM
3.4. DLP
3.5. Electrospinning
3.6. Others
4. Research Progress of Shape Memory Materials in Bone Histology
4.1. Shape Memory Polymer
4.2. Shape Memory Hydrogel
4.3. Shape Memory Alloys
4.4. Composite Shape Memory Material
4.4.1. Polymer Plus Polymer
4.4.2. Polymer Plus Hydrogel
4.4.3. Polymer Plus Ceramics
5. Discussion and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASC | adipose-derived mesenchymal stem cell |
| BMP-2 | bone morphogenetic protein-2 |
| BMSC | bone marrow mesenchymal stem cells |
| CS | Chitosan |
| DIW | direct ink writing |
| DLP | Direct Light Printing |
| FDM | fused deposition modeling |
| Gel | gelatin |
| GelMA | gelatin methacrylate |
| HAp | hydroxyapatite particles |
| PANI | Polyaniline |
| PCL/PCLDA | poly(ε-caprolactone)/poly(ε-caprolactone)-diacrylates |
| PD | polydopamine |
| PGS | poly (glycerol sebacate) |
| PLA/PLLA/PDLLA | Poly(lactic acid)/poly-L-lactic acid/poly(DL-lactic acid) |
| PPS | poly (1,3-propylene sebacate) |
| PU | polyurethane |
| RGD | arginyl-glycyl-aspartic acid |
| SLA | stereolithography |
| SLM/SLS | selective laser melting/ selective laser sintering |
| SMA/SMC/SMH/SMM/SMP | shape memory alloy ceramics/hydrogel/material/polymer |
| SPIO | superparamagnetic iron oxide |
| Ttrans | shape memory transition temperature |
References
- Qasim, M.; Chae, D.S.; Lee, N.Y. Advancements and frontiers in nano-based 3D and 4D scaffolds for bone and cartilage tissue engineering. Int. J. Nanomed. 2019, 14, 4333–4351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, M.; Wang, C.; Zhou, X.; Libanori, A.; Jiang, X.; Xu, W.; Zhu, S.; Chen, Q.; Sun, W.; Khademhosseini, A. Multi-Dimensional Printing for Bone Tissue Engineering. Adv. Healthc. Mater. 2021, 10, e2001986. [Google Scholar] [CrossRef] [PubMed]
- Amirazad, H.; Dadashpour, M.; Zarghami, N. Application of decellularized bone matrix as a bioscaffold in bone tissue engineering. J. Biol. Eng. 2022, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Ashammakhi, N.; Hasan, A.; Kaarela, O.; Byambaa, B.; Sheikhi, A.; Gaharwar, A.K.; Khademhosseini, A. Advancing Frontiers in Bone Bioprinting. Adv. Healthc. Mater. 2019, 8, e1801048. [Google Scholar] [CrossRef] [PubMed]
- Khademhosseini, A.; Langer, R. A decade of progress in tissue engineering. Nat. Protoc. 2016, 11, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, C.J.; Saul, J.M. Biomaterials for the Delivery of Growth Factors and Other Therapeutic Agents in Tissue Engineering Approaches to Bone Regeneration. Front. Pharm. 2018, 9, 513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.S.; Kim, J.H.; Jeong, J.; Kim, S.H.L.; Koh, R.H.; Kim, I.; Bae, S.; Lee, H.; Hwang, N.S. Sequential growth factor releasing double cryogel system for enhanced bone regeneration. Biomaterials 2020, 257, 120223. [Google Scholar] [CrossRef]
- Jin, Y.-Z.; Lee, J.H. Mesenchymal Stem Cell Therapy for Bone Regeneration. Clin. Orthop. Surg. 2018, 10, 271–278. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Mozafari, M.; Mehraien, M.; Vashaee, D.; Tayebi, L. Electroconductive Nanocomposite Scaffolds: A New Strategy Into Tissue Engineering and Regenerative Medicine. In Nanocomposites—New Trends and Developments; IntechOpen: London, UK, 2012; pp. 369–392. [Google Scholar]
- Kantaros, A. 3D Printing in Regenerative Medicine: Technologies and Resources Utilized. Int. J. Mol. Sci. 2022, 23, 14621. [Google Scholar] [CrossRef]
- Patty, D.J.; Nugraheni, A.D.; Dewi Ana, I.; Yusuf, Y. Mechanical Characteristics and Bioactivity of Nanocomposite Hydroxyapatite/Collagen Coated Titanium for Bone Tissue Engineering. Bioengineering 2022, 9, 784. [Google Scholar] [CrossRef] [PubMed]
- Kantaros, A. Bio-Inspired Materials: Exhibited Characteristics and Integration Degree in Bio-Printing Operations. Am. J. Eng. Appl. Sci. 2022, 15, 255–263. [Google Scholar] [CrossRef]
- Kuang, X.; Roach, D.J.; Wu, J.; Hamel, C.M.; Ding, Z.; Wang, T.; Dunn, M.L.; Qi, H.J. Advances in 4D Printing Materials and Applications. Adv. Funct. Mater. 2018, 29, 1805290. [Google Scholar] [CrossRef]
- Saska, S.; Pilatti, L.; Blay, A.; Shibli, J.A. Bioresorbable Polymers: Advanced Materials and 4D Printing for Tissue Engineering. Polymers 2021, 13, 563. [Google Scholar] [CrossRef]
- Jo, Y.; Hwang, S.H.; Jang, J. Employing Extracellular Matrix-Based Tissue Engineering Strategies for Age-Dependent Tissue Degenerations. Int. J. Mol. Sci. 2021, 22, 9367. [Google Scholar] [CrossRef]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- Li, Y.C.; Zhang, Y.S.; Akpek, A.; Shin, S.R.; Khademhosseini, A. 4D bioprinting: The next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication 2016, 9, 012001. [Google Scholar] [CrossRef] [Green Version]
- Ryplida, B.; Lee, K.D.; In, I.; Park, S.Y. Light-Induced Swelling-Responsive Conductive, Adhesive, and Stretchable Wireless Film Hydrogel as Electronic Artificial Skin. Adv. Funct. Mater. 2019, 29, 1903209. [Google Scholar] [CrossRef]
- Sreedhar, M.; Kalyana Chakravarthy, Y. Design and Evaluation of a Microsensor for a Bionic Hand with Metamaterials. Innovations in Mechanical Engineering; Springer Link: Guntur, India, 2022; pp. 699–709. [Google Scholar]
- Lohse, F.; Wende, C.; Klass, K.-D.; Hickmann, R.; Häntzsche, E.; Bollengier, Q.; Ashir, M.; Pöschel, R.; Bolk, N.; Trümper, W.; et al. Bio-inspired semi-flexible joint based on fibre-reinforced composites with shape memory alloys. J. Intell. Mater. Syst. Struct. 2020, 32, 462–472. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Y.; He, X.; Li, H.; Chen, Y.; Wei, Y.; Zhao, Y.; Ma, Y.; Chen, Z.; Zheng, X.; et al. Multiresponse Shape-Memory Nanocomposite with a Reversible Cycle for Powerful Artificial Muscles. Chem. Mater. 2021, 33, 987–997. [Google Scholar] [CrossRef]
- Gong, N.; Jin, H.; Sun, S.; Mao, S.; Li, W.; Zhang, S. A bionic soft tongue driven by shape memory alloy and pneumatics. Bioinspir. Biomim. 2021, 16, 055008. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz Memiş, N.; Kaplan, S. Production of thermal and water responsive shape memory polyurethane nanocomposite filaments with cellulose nanowhisker incorporation. Cellulose 2021, 28, 7075–7096. [Google Scholar] [CrossRef]
- Abdollahi, A.; Roghani-Mamaqani, H.; Razavi, B.; Salami-Kalajahi, M. The light-controlling of temperature-responsivity in stimuli-responsive polymers. Polym. Chem. 2019, 10, 5686–5720. [Google Scholar] [CrossRef]
- Herath, M.; Epaarachchi, J.; Islam, M.; Fang, L.; Leng, J. Light activated shape memory polymers and composites: A review. Eur. Polym. J. 2020, 136, 109912. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Heydarpour, R.; Tehrani, Z.M. Multi-stimuli-responsive hydrogels and their medical applications. New J. Chem. 2021, 45, 15705–15717. [Google Scholar] [CrossRef]
- Nath, J.; Chowdhury, A.; Dolui, S.K. Chitosan/graphene oxide-based multifunctional pH-responsive hydrogel with significant mechanical strength, self-healing property, and shape memory effect. Adv. Polym. Technol. 2018, 37, 3665–3679. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.; Gray, D.M.; Niezabitowska, E.; McDonald, T.O. Multi-stimuli-responsive aggregation of nanoparticles driven by the manipulation of colloidal stability. Nanoscale 2021, 13, 7879–7896. [Google Scholar] [CrossRef]
- Buffington, S.L.; Paul, J.E.; Ali, M.M.; Macios, M.M.; Mather, P.T.; Henderson, J.H. Enzymatically triggered shape memory polymers. Acta Biomater. 2019, 84, 88–97. [Google Scholar] [CrossRef]
- Subash, A.; Kandasubramanian, B. 4D printing of shape memory polymers. Eur. Polym. J. 2020, 134, 109771. [Google Scholar] [CrossRef]
- Melocchi, A.; Uboldi, M.; Cerea, M.; Foppoli, A.; Maroni, A.; Moutaharrik, S.; Palugan, L.; Zema, L.; Gazzaniga, A. Shape memory materials and 4D printing in pharmaceutics. Adv. Drug Deliv. Rev. 2021, 173, 216–237. [Google Scholar] [CrossRef]
- Miao, S.; Castro, N.; Nowicki, M.; Xia, L.; Cui, H.; Zhou, X.; Zhu, W.; Lee, S.J.; Sarkar, K.; Vozzi, G.; et al. 4D printing of polymeric materials for tissue and organ regeneration. Mater. Today 2017, 20, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Tibbits, S. 4D Printing: Multi-Material Shape Change. Archit. Des. 2014, 84, 116–121. [Google Scholar] [CrossRef]
- Ahmed, K.; Shiblee, M.D.N.I.; Khosla, A.; Nagahara, L.; Thundat, T.; Furukawa, H. Review—Recent Progresses in 4D Printing of Gel Materials. J. Electrochem. Soc. 2020, 167, 037563. [Google Scholar] [CrossRef]
- Zhang, Z.; Demir, K.G.; Gu, G.X. Developments in 4D-printing: A review on current smart materials, technologies, and applications. Int. J. Smart Nano Mater. 2019, 10, 205–224. [Google Scholar] [CrossRef] [Green Version]
- Afzali Naniz, M.; Askari, M.; Zolfagharian, A.; Afzali Naniz, M.; Bodaghi, M. 4D printing: A cutting-edge platform for biomedical applications. Biomed. Mater. 2022, 17, 062001. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Vimalraj, S.; Thanikaivelan, P.; Banudevi, S.; Manivasagam, G. A review on injectable chitosan/beta glycerophosphate hydrogels for bone tissue regeneration. Int. J. Biol. Macromol. 2019, 121, 38–54. [Google Scholar] [CrossRef]
- Senatov, F.S.; Niaza, K.V.; Zadorozhnyy, M.Y.; Maksimkin, A.V.; Kaloshkin, S.D.; Estrin, Y.Z. Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds. J. Mech. Behav. Biomed. Mater. 2016, 57, 139–148. [Google Scholar] [CrossRef]
- Graham, S.; Marina, P.F.; Blencowe, A. Thermoresponsive polysaccharides and their thermoreversible physical hydrogel networks. Carbohydr. Polym. 2019, 207, 143–159. [Google Scholar] [CrossRef]
- Hoa, S.V.; Cai, X. Twisted composite structures made by 4D printing method. Compos. Struct. 2020, 238, 111883. [Google Scholar] [CrossRef]
- Jeong, H.Y.; An, S.-C.; Lim, Y.; Jeong, M.J.; Kim, N.; Jun, Y.C. 3D and 4D Printing of Multistable Structures. Appl. Sci. 2020, 10, 7254. [Google Scholar] [CrossRef]
- Wang, Z.; Agrawal, P.; Zhang, Y.S. Nanotechnologies and Nanomaterials in 3D (Bio)printing toward Bone Regeneration. Adv. NanoBiomed Res. 2021, 1, 2100035. [Google Scholar] [CrossRef]
- Wang, C.; Yue, H.; Liu, J.; Zhao, Q.; He, Z.; Li, K.; Lu, B.; Huang, W.; Wei, Y.; Tang, Y.; et al. Advanced reconfigurable scaffolds fabricated by 4D printing for treating critical-size bone defects of irregular shapes. Biofabrication 2020, 12, 045025. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Amirthalingam, S.; Kim, S.L.; Lee, S.S.; Rangasamy, J.; Hwang, N.S. Biomimetic Materials and Fabrication Approaches for Bone Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700612. [Google Scholar] [CrossRef] [PubMed]
- Vaithilingam, J.; Sanjuan-Alberte, P.; Campora, S.; Rance, G.A.; Jiang, L.; Thorpe, J.; Burroughs, L.; Tuck, C.J.; Denning, C.; Wildman, R.D.; et al. Multifunctional Bioinstructive 3D Architectures to Modulate Cellular Behavior. Adv. Funct. Mater. 2019, 29, 1902016. [Google Scholar] [CrossRef] [Green Version]
- Khorsandi, D.; Fahimipour, A.; Abasian, P.; Saber, S.S.; Seyedi, M.; Ghanavati, S.; Ahmad, A.; De Stephanis, A.A.; Taghavinezhaddilami, F.; Leonova, A.; et al. 3D and 4D printing in dentistry and maxillofacial surgery: Printing techniques, materials, and applications. Acta Biomater. 2021, 122, 26–49. [Google Scholar] [CrossRef]
- Kumari, G.; Abhishek, K.; Singh, S.; Hussain, A.; Altamimi, M.A.; Madhyastha, H.; Webster, T.J.; Dev, A. A voyage from 3D to 4D printing in nanomedicine and healthcare: Part I. Nano Med. 2022, 17, 237–253. [Google Scholar]
- Kamio, T.; Onda, T. Fused Deposition Modeling 3D Printing in Oral and Maxillofacial Surgery: Problems and Solutions. Cureus 2022, 14, e28906. [Google Scholar] [CrossRef]
- Velasquez-Garcia, L.F.; Kornbluth, Y. Biomedical Applications of Metal 3D Printing. Annu. Rev. Biomed. Eng. 2021, 23, 307–338. [Google Scholar] [CrossRef]
- Xu, W.; Jambhulkar, S.; Zhu, Y.; Ravichandran, D.; Kakarla, M.; Vernon, B.; Lott, D.G.; Cornella, J.L.; Shefi, O.; Miquelard-Garnier, G.; et al. 3D printing for polymer/particle-based processing: A review. Compos. Part B Eng. 2021, 223, 109102. [Google Scholar] [CrossRef]
- Wolf, A.; Rosendahl, P.L.; Knaack, U. Additive manufacturing of clay and ceramic building components. Autom. Constr. 2022, 133, 103956. [Google Scholar] [CrossRef]
- Ferroni, L.; D’Amora, U.; Leo, S.; Tremoli, E.; Raucci, M.G.; Ronca, A.; Ambrosio, L.; Zavan, B. PEEK and Hyaluronan-Based 3D Printed Structures: Promising Combination to Improve Bone Regeneration. Molecules 2022, 27, 8749. [Google Scholar] [CrossRef] [PubMed]
- Kantaros, A.; Piromalis, D. Fabricating Lattice Structures via 3D Printing: The Case of Porous Bio-Engineered Scaffolds. Appl. Mech. 2021, 2, 289–302. [Google Scholar] [CrossRef]
- Shakibania, S.; Ghazanfari, L.; Raeeszadeh-Sarmazdeh, M.; Khakbiz, M. Medical application of biomimetic 4D printing. Drug Dev. Ind. Pharm. 2021, 47, 521–534. [Google Scholar] [CrossRef]
- Elder, B.; Neupane, R.; Tokita, E.; Ghosh, U.; Hales, S.; Kong, Y.L. Nanomaterial Patterning in 3D Printing. Adv. Mater. 2020, 32, e1907142. [Google Scholar] [CrossRef]
- Park, S.; Fu, K. Polymer-based filament feedstock for additive manufacturing. Compos. Sci. Technol. 2021, 213, 108876. [Google Scholar] [CrossRef]
- Fina, F.; Gaisford, S.; Basit, A.W. Powder Bed Fusion: The Working Process, Current Applications and Opportunities. 3D Printing of Pharmaceuticals; Springer Link: London, UK, 2018; pp. 81–105. [Google Scholar]
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl. Mater. Today 2020, 18, 100479. [Google Scholar] [CrossRef]
- Momeni, F.; M.Mehdi Hassani.N, S.; Liu, X.; Ni, J. A review of 4D printing. Mater. Des. 2017, 122, 42–79. [Google Scholar] [CrossRef]
- Morouço, P.; Azimi, B.; Milazzo, M.; Mokhtari, F.; Fernandes, C.; Reis, D.; Danti, S. Four-Dimensional (Bio-)printing: A Review on Stimuli-Responsive Mechanisms and Their Biomedical Suitability. Appl. Sci. 2020, 10, 9143. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Significant roles of 4D printing using smart materials in the field of manufacturing. Adv. Ind. Eng. Polym. Res. 2021, 4, 301–311. [Google Scholar] [CrossRef]
- Quan, H.; Kisailus, D.; Meyers, M.A. Hydration-induced reversible deformation of biological materials. Nat. Rev. Mater. 2020, 6, 264–283. [Google Scholar] [CrossRef]
- Apsite, I.; Biswas, A.; Li, Y.; Ionov, L. Microfabrication Using Shape-Transforming Soft Materials. Adv. Funct. Mater. 2020, 30, 1908028. [Google Scholar] [CrossRef]
- Stoychev, G.; Kirillova, A.; Ionov, L. Light-Responsive Shape-Changing Polymers. Adv. Opt. Mater. 2019, 7, 1900067. [Google Scholar] [CrossRef]
- Le Fer, G.; Becker, M.L. 4D Printing of Resorbable Complex Shape-Memory Poly(propylene fumarate) Star Scaffolds. ACS Appl. Mater. Interfaces 2020, 12, 22444–22452. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lin, X.; Chen, B.; Wei, X. Cell-laden four-dimensional bioprinting using near-infrared-triggered shape-morphing alginate/polydopamine bioinks. Biofabrication 2019, 11, 045019. [Google Scholar] [CrossRef]
- Liu, X.; Chen, W.; Shao, B.; Zhang, X.; Wang, Y.; Zhang, S.; Wu, W. Mussel patterned with 4D biodegrading elastomer durably recruits regenerative macrophages to promote regeneration of craniofacial bone. Biomaterials 2021, 276, 120998. [Google Scholar] [CrossRef]
- Wang, J.; Gao, H.; Hu, Y.; Zhang, N.; Zhou, W.; Wang, C.; Binks, B.P.; Yang, Z. 3D printing of Pickering emulsion inks to construct poly(D,L-lactide-co-trimethylene carbonate)-based porous bioactive scaffolds with shape memory effect. J. Mater. Sci. 2020, 56, 731–745. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, D.; Liao, P.; Su, J.W.; Deng, H.; Vardhanabhuti, B.; Ulery, B.D.; Chen, S.Y.; Lin, J. 4D Printing of shape-memory polymeric scaffolds for adaptive biomedical implantation. Acta Biomater. 2021, 122, 101–110. [Google Scholar] [CrossRef]
- Kirillova, A.; Maxson, R.; Stoychev, G.; Gomillion, C.T.; Ionov, L. 4D Biofabrication Using Shape-Morphing Hydrogels. Adv. Mater. 2017, 29, 1703443. [Google Scholar] [CrossRef]
- Lai, J.; Ye, X.; Liu, J.; Wang, C.; Li, J.; Wang, X.; Ma, M.; Wang, M. 4D printing of highly printable and shape morphing hydrogels composed of alginate and methylcellulose. Mater. Des. 2021, 205, 109699. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Ye, J.H.; Fu, J.Z.; Gao, Q.; He, Y. 4D Printing of High-Performance Thermal-Responsive Liquid Metal Elastomers Driven by Embedded Microliquid Chambers. ACS Appl. Mater. Interfaces 2020, 12, 12068–12074. [Google Scholar] [CrossRef]
- Ding, A.; Jeon, O.; Cleveland, D.; Gasvoda, K.L.; Wells, D.; Lee, S.J.; Alsberg, E. Jammed Micro-Flake Hydrogel for Four-Dimensional Living Cell Bioprinting. Adv. Mater. 2022, 34, e2109394. [Google Scholar] [CrossRef] [PubMed]
- Diba, M.; Koons, G.L.; Bedell, M.L.; Mikos, A.G. 3D printed colloidal biomaterials based on photo-reactive gelatin nanoparticles. Biomaterials 2021, 274, 120871. [Google Scholar] [CrossRef] [PubMed]
- Langford, T.; Mohammed, A.; Essa, K.; Elshaer, A.; Hassanin, H. 4D Printing of Origami Structures for Minimally Invasive Surgeries Using Functional Scaffold. Appl. Sci. 2020, 11, 332. [Google Scholar] [CrossRef]
- Yue, C.; Li, M.; Liu, Y.; Fang, Y.; Song, Y.; Xu, M.; Li, J. Three-dimensional printing of cellulose nanofibers reinforced PHB/PCL/Fe3O4 magneto-responsive shape memory polymer composites with excellent mechanical properties. Addit. Manuf. 2021, 46, 102146. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, G.; Singh, S.; Jha, K.; Prakash, C. 3D printed biodegradable functional temperature-stimuli shape memory polymer for customized scaffoldings. J. Mech. Behav. Biomed. Mater. 2020, 108, 103781. [Google Scholar] [CrossRef]
- Singh, G.; Singh, S.; Prakash, C.; Kumar, R.; Kumar, R.; Ramakrishna, S. Characterization of three-dimensional printed thermal-stimulus polylactic acid-hydroxyapatite-based shape memory scaffolds. Polym. Compos. 2020, 41, 3871–3891. [Google Scholar] [CrossRef]
- Wang, Y.J.; Jeng, U.S.; Hsu, S.H. Biodegradable Water-Based Polyurethane Shape Memory Elastomers for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 1397–1406. [Google Scholar] [CrossRef]
- Miao, S.; Cui, H.; Esworthy, T.; Mahadik, B.; Lee, S.J.; Zhou, X.; Hann, S.Y.; Fisher, J.P.; Zhang, L.G. 4D Self-Morphing Culture Substrate for Modulating Cell Differentiation. Adv. Sci. 2020, 7, 1902403. [Google Scholar] [CrossRef] [Green Version]
- Naujokat, H.; Gokkaya, A.I.; Acil, Y.; Loger, K.; Kluter, T.; Fuchs, S.; Wiltfang, J. In vivo biocompatibility evaluation of 3D-printed nickel-titanium fabricated by selective laser melting. J. Mater. Sci. Mater. Med. 2022, 33, 13. [Google Scholar] [CrossRef]
- Saedi, S.; Saghaian, S.E.; Jahadakbar, A.; Shayesteh Moghaddam, N.; Taheri Andani, M.; Saghaian, S.M.; Lu, Y.C.; Elahinia, M.; Karaca, H.E. Shape memory response of porous NiTi shape memory alloys fabricated by selective laser melting. J. Mater. Sci. Mater. Med. 2018, 29, 40. [Google Scholar] [CrossRef]
- Kim, S.H.; Seo, Y.B.; Yeon, Y.K.; Lee, Y.J.; Park, H.S.; Sultan, M.T.; Lee, J.M.; Lee, J.S.; Lee, O.J.; Hong, H.; et al. 4D-bioprinted silk hydrogels for tissue engineering. Biomaterials 2020, 260, 120281. [Google Scholar] [CrossRef] [PubMed]
- You, D.; Chen, G.; Liu, C.; Ye, X.; Wang, S.; Dong, M.; Sun, M.; He, J.; Yu, X.; Ye, G.; et al. 4D Printing of Multi-Responsive Membrane for Accelerated In Vivo Bone Healing Via Remote Regulation of Stem Cell Fate. Adv. Funct. Mater. 2021, 31, 2103920. [Google Scholar] [CrossRef]
- Wang, X.; Yan, H.; Shen, Y.; Tang, H.; Yi, B.; Qin, C.; Zhang, Y. Shape Memory and Osteogenesis Capabilities of the Electrospun Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Modified Poly(l-Lactide) Fibrous Mats. Tissue Eng. Part A 2021, 27, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Tang, D.; Sun, Z.; Gao, J.; Yang, X.; Jia, S.; Peng, J. Electrospun PCL-based polyurethane/HA microfibers as drug carrier of dexamethasone with enhanced biodegradability and shape memory performances. Colloid Polym. Sci. 2019, 298, 103–111. [Google Scholar] [CrossRef]
- Miao, S.; Zhu, W.; Castro, N.J.; Nowicki, M.; Zhou, X.; Cui, H.; Fisher, J.P.; Zhang, L.G. 4D printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate. Sci. Rep. 2016, 6, 27226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constante, G.; Apsite, I.; Alkhamis, H.; Dulle, M.; Schwarzer, M.; Caspari, A.; Synytska, A.; Salehi, S.; Ionov, L. 4D Biofabrication Using a Combination of 3D Printing and Melt-Electrowriting of Shape-Morphing Polymers. ACS Appl. Mater. Interfaces 2021, 13, 12767–12776. [Google Scholar] [CrossRef]
- Alshahrani, H.A. Review of 4D printing materials and reinforced composites: Behaviors, applications and challenges. J. Sci. Adv. Mater. Devices 2021, 6, 167–185. [Google Scholar] [CrossRef]
- Mehta, P.; Sahlot, P. Application of phase change materials in 4D printing: A review. Mater. Today Proc. 2021, 47, 4746–4752. [Google Scholar] [CrossRef]
- Sobacchi, C.; Erreni, M.; Strina, D.; Palagano, E.; Villa, A.; Menale, C. 3D Bone Biomimetic Scaffolds for Basic and Translational Studies with Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 3150. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, A.; Baigent, A.; Raghav, S.; Brádaigh, C.Ó.; Koutsos, V.; Radacsi, N. 4D Printing: Materials, Technologies, and Future Applications in the Biomedical Field. Sustainability 2020, 12, 10628. [Google Scholar] [CrossRef]
- Bhanushali, H.; Amrutkar, S.; Mestry, S.; Mhaske, S.T. Shape memory polymer nanocomposite: A review on structure–property relationship. Polym. Bull. 2021, 79, 3437–3493. [Google Scholar] [CrossRef]
- Zare, M.; Davoodi, P.; Ramakrishna, S. Electrospun Shape Memory Polymer Micro-/Nanofibers and Tailoring Their Roles for Biomedical Applications. Nanomaterials 2021, 11, 933. [Google Scholar] [CrossRef] [PubMed]
- Shiblee, M.D.N.I.; Ahmed, K.; Kawakami, M.; Furukawa, H. 4D Printing of Shape-Memory Hydrogels for Soft-Robotic Functions. Adv. Mater. Technol. 2019, 4, 1900071. [Google Scholar] [CrossRef]
- Park, J.; Park, S.Y.; Lee, D.; Song, Y.S. Shape memory polymer composites embedded with hybrid ceramic microparticles. Smart Mater. Struct. 2020, 29, 055037. [Google Scholar] [CrossRef]
- Urbina, L.; Alonso-Varona, A.; Saralegi, A.; Palomares, T.; Eceiza, A.; Corcuera, M.A.; Retegi, A. Hybrid and biocompatible cellulose/polyurethane nanocomposites with water-activated shape memory properties. Carbohydr. Polym. 2019, 216, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Erndt-Marino, J.D.; Munoz-Pinto, D.J.; Samavedi, S.; Jimenez-Vergara, A.C.; Diaz-Rodriguez, P.; Woodard, L.; Zhang, D.; Grunlan, M.A.; Hahn, M.S. Evaluation of the Osteoinductive Capacity of Polydopamine-Coated Poly(epsilon-caprolactone) Diacrylate Shape Memory Foams. ACS Biomater. Sci. Eng. 2015, 1, 1220–1230. [Google Scholar] [CrossRef]
- Pfau, M.R.; Beltran, F.O.; Woodard, L.N.; Dobson, L.K.; Gasson, S.B.; Robbins, A.B.; Lawson, Z.T.; Brian Saunders, W.; Moreno, M.R.; Grunlan, M.A. Evaluation of a self-fitting, shape memory polymer scaffold in a rabbit calvarial defect model. Acta Biomater. 2021, 136, 233–242. [Google Scholar] [CrossRef]
- Yang, W.; Guan, D.; Liu, J.; Luo, Y.; Wang, Y. Synthesis and characterization of biodegradable linear shape memory polyurethanes with high mechanical performance by incorporating novel long chain diisocyanates. New J. Chem. 2020, 44, 3493–3503. [Google Scholar] [CrossRef]
- Guo, Y.; Lv, Z.; Huo, Y.; Sun, L.; Chen, S.; Liu, Z.; He, C.; Bi, X.; Fan, X.; You, Z. A biodegradable functional water-responsive shape memory polymer for biomedical applications. J. Mater. Chem. B 2019, 7, 123–132. [Google Scholar] [CrossRef]
- Ying, Y.; Li, B.; Liu, C.; Xiong, Z.; Bai, W.; Ma, P. Shape-Memory ECM-Mimicking Heparin-Modified Nanofibrous Gelatin Scaffold for Enhanced Bone Regeneration in Sinus Augmentation. ACS Biomater. Sci. Eng. 2022, 8, 218–231. [Google Scholar] [CrossRef]
- Yuan, Z.; Yuan, X.; Zhao, Y.; Cai, Q.; Wang, Y.; Luo, R.; Yu, S.; Wang, Y.; Han, J.; Ge, L.; et al. Injectable GelMA Cryogel Microspheres for Modularized Cell Delivery and Potential Vascularized Bone Regeneration. Small 2021, 17, e2006596. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.B.; Su, D.H.; Liu, P.; Ma, Y.Q.; Shao, Z.Z.; Dong, J. Shape-memory collagen scaffold for enhanced cartilage regeneration: Native collagen versus denatured collagen. Osteoarthr. Cartil. 2018, 26, 1389–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, J.P.Y.; Samartzis, D.; Yeung, K.; To, M.; Luk, K.D.K.; Cheung, K.M. A randomized double-blinded clinical trial to evaluate the safety and efficacy of a novel superelastic nickel-titanium spinal rod in adolescent idiopathic scoliosis: 5-year follow-up. Eur. Spine J. 2018, 27, 327–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Riet, R.P.; Bain, G.I. Three-corner Wrist Fusion Using Memory Staples. Tech. Hand Up. Extrem. Surg. 2006, 10, 259–264. [Google Scholar] [CrossRef]
- Werner, M.; Hammer, N.; Rotsch, C.; Berthold, I.; Leimert, M. Experimental validation of adaptive pedicle screws-a novel implant concept using shape memory alloys. Med. Biol. Eng. Comput. 2020, 58, 55–65. [Google Scholar] [CrossRef]
- Zakaria, O.; Madi, M.; Kasugai, S. Introduction of a novel guided bone regeneration memory shape based device. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 460–467. [Google Scholar] [CrossRef]
- De Vitis, R.; Passiatore, M.; Perna, A.; Fioravanti Cinci, G.; Taccardo, G. Comparison of Shape Memory Staple and Gelled Platelet-Rich Plasma versus Shape Memory Staple alone for the Treatment of Waist Scaphoid Nonunion: A Single-Center Experience. Joints 2019, 7, 84–90. [Google Scholar] [CrossRef]
- Hou, Z.; Liu, Z.; Zhu, X.; Xie, Y.; Yan, F.; Yin, H.; Zhang, Z.; Wu, M.; Liang, X.; Deng, Z.; et al. Contactless treatment for scoliosis by electromagnetically controlled shape-memory alloy rods: A preliminary study in rabbits. Eur. Spine J. 2020, 29, 1147–1158. [Google Scholar] [CrossRef]
- Akbarinia, S.; Sadrnezhaad, S.K.; Hosseini, S.A. Porous shape memory dental implant by reactive sintering of TiH2-Ni-Urea mixture. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110213. [Google Scholar] [CrossRef]
- Liu, J.; Lin, Y.; Bian, D.; Wang, M.; Lin, Z.; Chu, X.; Li, W.; Liu, Y.; Shen, Z.; Liu, Y.; et al. In vitro and in vivo studies of Mg-30Sc alloys with different phase structure for potential usage within bone. Acta Biomater. 2019, 98, 50–66. [Google Scholar] [CrossRef]
- Rajeshkumar, G.; Arvindh Seshadri, S.; Devnani, G.L.; Sanjay, M.R.; Siengchin, S.; Prakash Maran, J.; Al-Dhabi, N.A.; Karuppiah, P.; Mariadhas, V.A.; Sivarajasekar, N.; et al. Environment friendly, renewable and sustainable poly lactic acid (PLA) based natural fiber reinforced composites—A comprehensive review. J. Clean. Prod. 2021, 310, 127483. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Ye, L.; Coates, P.; Caton-Rose, F. Multiple shape memory behavior of highly oriented long-chain-branched poly(lactic acid) and its recovery mechanism. J. Biomed. Mater. Res. A 2019, 107, 872–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuai, L.; Jun, Z.; Jianjun, C.; Ming, Y.; Xuepeng, L.; Zhiguo, J. Biodegradable body temperature-responsive shape memory polyurethanes with self-healing behavior. Polym. Eng. Sci. 2019, 59, E310–E316. [Google Scholar] [CrossRef]
- Joshi, S.; Rawat, K.; Karunakaran, C.; Rajamohan, V.; Mathew, A.T.; Koziol, K.; Kumar Thakur, V.; Balan, A.S.S. 4D printing of materials for the future: Opportunities and challenges. Appl. Mater. Today 2020, 18, 100490. [Google Scholar] [CrossRef]
- Zhou, P.Y.; Jiang, L.Q.; Xia, D.M.; Wu, J.H.; Ye, Y.; Xu, S.G. Nickel-titanium arched shape-memory alloy connector combined with bone grafting in the treatment of scaphoid nonunion. Eur. J. Med. Res. 2019, 24, 27. [Google Scholar] [CrossRef] [Green Version]
- Hamann, I.; Gebhardt, F.; Eisenhut, M.; Koch, P.; Thielsch, J.; Rotsch, C.; Drossel, W.G.; Heyde, C.E.; Leimert, M. Investigation into the Hybrid Production of a Superelastic Shape Memory Alloy with Additively Manufactured Structures for Medical Implants. Materials 2021, 14, 3098. [Google Scholar] [CrossRef]
- Takale, A.M.; Chougule, N.K. Effect of wire electro discharge machining process parameters on surface integrity of Ti49.4Ni50.6 shape memory alloy for orthopedic implant application. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 264–274. [Google Scholar] [CrossRef]
- Srivastava, S.; Bhalla, S.; Madan, A. Shape memory alloy actuation of non-bonded piezo sensor configuration for bone diagnosis and impedance based analysis. Biomed. Eng. Lett. 2019, 9, 435–447. [Google Scholar] [CrossRef]
- Zhukova, P.A.; Senatov, F.S.; Zadorozhnyy, M.Y.; Chmelyuk, N.S.; Zaharova, V.A. Polymer Composite Materials Based on Polylactide with a Shape Memory Effect for “Self-Fitting” Bone Implants. Polymers 2021, 13, 2367. [Google Scholar] [CrossRef]
- Arabiyat, A.S.; Pfau, M.R.; Grunlan, M.A.; Hahn, M.S. Intrinsic osteoinductivity of PCL-DA/PLLA semi-IPN shape memory polymer scaffolds. J. Biomed. Mater. Res. A 2021, 109, 2334–2345. [Google Scholar] [CrossRef] [PubMed]
- Shaabani, A.; Sedghi, R.; Motasadizadeh, H.; Dinarvand, R. Self-healable conductive polyurethane with the body temperature-responsive shape memory for bone tissue engineering. Chem. Eng. J. 2021, 411, 128449. [Google Scholar] [CrossRef]
- Xuan, H.; Hu, H.; Geng, C.; Song, J.; Shen, Y.; Lei, D.; Guan, Q.; Zhao, S.; You, Z. Biofunctionalized chondrogenic shape-memory ternary scaffolds for efficient cell-free cartilage regeneration. Acta Biomater. 2020, 105, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; He, J.; Yong, X.; Lu, J.; Xiao, J.; Liao, Y.; Li, Q.; Xiong, C. Biodegradable poly (lactic acid-co-trimethylene carbonate)/chitosan microsphere scaffold with shape-memory effect for bone tissue engineering. Colloids Surf. B Biointerfaces 2020, 195, 111218. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.; Lee, S.J.; Ayyagari, S.; Tang, R.; Huynh, C.T.; Alsberg, E. 4D biofabrication via instantly generated graded hydrogel scaffolds. Bioact. Mater. 2022, 7, 324–332. [Google Scholar] [CrossRef]
- Shaabani, A.; Sedghi, R. Preparation of chitosan biguanidine/PANI-containing self-healing semi-conductive waterborne scaffolds for bone tissue engineering. Carbohydr. Polym. 2021, 264, 118045. [Google Scholar] [CrossRef]
- Wu, S.D.; Hsu, S.H. 4D bioprintable self-healing hydrogel with shape memory and cryopreserving properties. Biofabrication 2021, 13, 045029. [Google Scholar] [CrossRef]
- Kim, S.E.; Park, K. Recent Advances of Biphasic Calcium Phosphate Bioceramics for Bone Tissue Regeneration. Adv. Exp. Med. Biol. 2020, 1250, 177–188. [Google Scholar]
- He, Z.; Liu, Y.; Liu, X.; Sun, Y.; Zhao, Q.; Liu, L.; Zhu, Z.; Luo, E. Smart Porous Scaffold Promotes Peri-Implant Osteogenesis under the Periosteum. ACS Biomater. Sci. Eng. 2020, 6, 6321–6330. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, K.; Gong, T.; Song, J.; Bao, C.; Luo, E.; Weng, J.; Zhou, S. Delivery of growth factors using a smart porous nanocomposite scaffold to repair a mandibular bone defect. Biomacromolecules 2014, 15, 1019–1030. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, X.; Yan, G.; Chen, X.; Luo, K.; Zhou, S.; Zhang, P.; Li, J.; Wong, T.W. Biomimetic scaffolds with programmable pore structures for minimum invasive bone repair. Nanoscale 2021, 13, 16680–16689. [Google Scholar] [CrossRef]
- Xie, R.; Hu, J.; Hoffmann, O.; Zhang, Y.; Ng, F.; Qin, T.; Guo, X. Self-fitting shape memory polymer foam inducing bone regeneration: A rabbit femoral defect study. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xia, H.; Teramoto, A.; Ni, Q.Q. The effect of hydroxyapatite nanoparticles on mechanical behavior and biological performance of porous shape memory polyurethane scaffolds. J. Biomed. Mater. Res. A 2018, 106, 244–254. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Zhao, X.; Xie, R.; Qin, T.; Ji, F. Mechanically Robust Shape Memory Polyurethane Nanocomposites for Minimally Invasive Bone Repair. ACS Appl. Bio Mater. 2019, 2, 1056–1065. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Xie, R.; Yang, Y.; Cao, J.; Tu, Y.; Zhang, Y.; Qin, T.; Zhao, X. A programmable, fast-fixing, osteo-regenerative, biomechanically robust bone screw. Acta Biomater. 2020, 103, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Imoto, K.; Yamauchi, K.; Odashima, K.; Nogami, S.; Shimizu, Y.; Kessler, P.; Lethaus, B.; Unuma, H.; Takahashi, T. Periosteal expansion osteogenesis using an innovative, shape-memory polyethylene terephthalate membrane: An experimental study in rabbits. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1327–1333. [Google Scholar] [CrossRef] [PubMed]



| Printing techniques | SLS, SLM, SLA, DLP, DIW, FDM, LOM, EBM |
| Smart behaviors | Shape memory, Self-assembly, Self-sensing, Self-healing |
| Stimulus | Physical (temperature, electrical, magnetic, light) |
| Chemical (redox, pH, ion, humidity) | |
| Biological (glucose, enzyme) | |
| Smart materials/bioinks | Naturally derived polymers (collagen, alginate, gelation) |
| Synthetic polymers (PLGA, PLA, PEG, PEO, PVA) |
| Classification | Material | Load | Stimulate | Biological Evaluation | Reference |
|---|---|---|---|---|---|
| SMP | PCLDA | PD | Temperature | In vitro: hMSCs | |
| In vivo: rabbit femoral defect | [99] | ||||
| In vivo: rabbit skull defect | [100] | ||||
| PU | Temperature | In vitro: osteoblasts | [101] | ||
| PGDA | Temperature | In vivo: mouse aorta | [70] | ||
| PBF | BMP-2 | Water | In vitro: osteoblasts | [102] | |
| Pellethane | Lipase | In vitro: fibroblasts | [30] | ||
| SMH | Gel | BMP-2 | Water | In vitro: rabbit BMSCs | |
| In vivo:rabbit maxillary sinus | [103] | ||||
| GelMA | Temperature | In vitro: hMSCs | |||
| In vivo:subcutaneous injection | [104] | ||||
| Col | Water | In vitro: rabbit chondrocytes | |||
| In vivo: rabbit knee joint | [105] | ||||
| Alginate | PD | Ca2+ | In vitro: mouse BMSCs | [71] | |
| SMA | Ni-Ti | Temperature | In vivo: adolescent scoliosis | [106] | |
| In vivo: scaphoid nonunion | [107] | ||||
| In vivo: vertebral model | [108] | ||||
| In vivo: subperiosteum | [109] | ||||
| Ni-Ti | PRP | Temperature | In vivo: scaphoid nonunion | [110] | |
| Ni-Ti | Magnetic | In vivo: rabbit scoliosis | [111] | ||
| Ni-TiH2 | Urea | Temperature | In vivo: palatine bone model | [112] | |
| Mg-Sc | Temperature | In vitro: MC3T3-E1 | |||
| In vivo: rat femoral defect | [113] |
| Classification | Material | Load | Stimulate | Biological Evaluation | Reference |
|---|---|---|---|---|---|
| SMP + SMP | PCL-PLLA | PD | Temperature | In vitro: hMSC | [123] |
| PU-AT | Temperature | In vitro: hASC | |||
| In vivo: rat skull | [124] | ||||
| PGS-PPS | Temperature | In vitro: rat BMSC | |||
| In vivo: rat knee cartilage | [125] | ||||
| PGS-PCL | PD | Temperature | In vitro:BMSC, macrophages | ||
| In vivo: rat skull, | [68] | ||||
| SMP + SMH | PLA-CS | Temperature | In vitro: MC3T3-E1 | [126] | |
| PCLDA- Gel | Temperature | In vitro: MSC | |||
| In vivo: rat femur | [85] | ||||
| PU-Gel | SPIO | Temperature | In vitro: hMSCs | [80] | |
| PANI-CS | CP | Temperature | In vitro: hADSCs | [128] | |
| SMP + SMC | PCL-HA | Temperature | In vitro: rabbit BMSCs | ||
| In vivo: subperiosteal | [131] | ||||
| BMP-2 | Temperature | In vitro: rabbit BMSCs | |||
| In vivo: rabbit mandible | [132] | ||||
| PU-HA | Temperature | In vitro: MC3T3-E1 | |||
| In vivo: rabbit femur | [134] | ||||
| RGD | Temperature | In vitro: rabbit MSC | [136,137] | ||
| PCL/PTMG-HA | Temperature | In vitro: MSC | |||
| PET-Gel-HA | Temperature | In vivo: subperiosteal | [138] | ||
| PDLLA -TCP | OP | NIR | In vitro: rat BMSC | ||
| In vivo: rat skull | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Yang, Y.; Yang, Z.; Ma, R.; Aimaijiang, M.; Xu, J.; Zhang, Y.; Zhou, Y. Four-Dimensional Printing and Shape Memory Materials in Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 814. https://doi.org/10.3390/ijms24010814
Zhang X, Yang Y, Yang Z, Ma R, Aimaijiang M, Xu J, Zhang Y, Zhou Y. Four-Dimensional Printing and Shape Memory Materials in Bone Tissue Engineering. International Journal of Molecular Sciences. 2023; 24(1):814. https://doi.org/10.3390/ijms24010814
Chicago/Turabian StyleZhang, Xinwei, Yixin Yang, Zhen Yang, Rui Ma, Maierhaba Aimaijiang, Jing Xu, Yidi Zhang, and Yanmin Zhou. 2023. "Four-Dimensional Printing and Shape Memory Materials in Bone Tissue Engineering" International Journal of Molecular Sciences 24, no. 1: 814. https://doi.org/10.3390/ijms24010814