Polymer Encapsulated Liposomes for Oral Co-Delivery of Curcumin and Hydroxytyrosol
Abstract
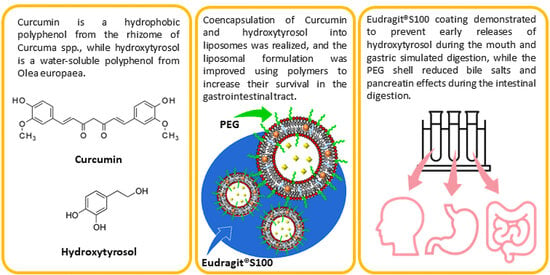
:1. Introduction
2. Results and Discussion
2.1. Liposome Preparation and Characterization
2.2. In Vitro Digestion
2.2.1. Liposome Characterization during the In Vitro Digestion
2.2.2. HT Release during the In Vitro Digestion
2.3. Antioxidant Activity
2.4. Cytobiocompatibility of Liposomes
3. Materials and Methods
3.1. Materials
3.2. Preparation of Liposomes
- Type 1: LS100 (4 mg/mL) + Chol (0.2 mg/mL, 10% mol);
- Type 2: LS100 (4 mg/mL) + Chol (0.2 mg/mL, 10% mol) + DSPE-PEG-2000 (1 mg/mL, 7% mol);
- Type 3: LS100 (4 mg/mL) + Chol (0.2 mg/mL, 10% mol) + DSPE-PEG-2000 (1 mg/mL, 7% mol) + EuS100 (2.5 mg/mL, 62.5% w/w).
3.3. Characterization of Liposomes
3.4. Simulated In Vitro Digestion
- Mouth phase: the simulated salivary fluid was composed of NaCl 8 mg/mL, α-amylase 1 mg/L, buffer KPi (2.5 mM), pH 6.75. The incubation was performed for 10 min;
- Gastric phase: the simulated gastric fluid is composed of pepsin 3.2 mg/mL, NaCl 0.03 M, pH 1.2. The incubation was performed for 120 min;
- Intestinal phase: the simulated intestinal fluid is composed of bile bovine 0.2 mg/mL, pancreatin 3.2 mg/mL, pH 7.4. The incubation was performed for 240 min.
3.5. Calcein Release Experiments
3.6. HT and Cur Release Experiments
3.7. Antioxidant Activity Assay
3.8. Cell Cultures and Cytotoxicity
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gan, R.-Y.; Chan, C.-L.; Yang, Q.-Q.; Li, H.-B.; Zhang, D.; Ge, Y.-Y.; Gunaratne, A.; Ge, J.; Corke, H. 9—Bioactive compounds and beneficial functions of sprouted grains. In Sprouted Grains; Feng, H., Nemzer, B., DeVries, J.W., Eds.; AACC International Press: Washington, DC, USA, 2019; pp. 191–246. [Google Scholar]
- González-Santiago, M.; Martín-Bautista, E.; Carrero, J.J.; Fonollá, J.; Baró, L.; Bartolomé, M.V.; Gil-Loyzaga, P.; López-Huertas, E. One-month administration of hydroxytyrosol, a phenolic antioxidant present in olive oil, to hyperlipemic rabbits improves blood lipid profile, antioxidant status and reduces atherosclerosis development. Atherosclerosis 2006, 188, 35–42. [Google Scholar] [CrossRef]
- Zafra-Gómez, A.; Luzón-Toro, B.; Capel-Cuevas, S.; Morales, J. Stability of Hydroxytyrosol in Aqueous Solutions at Different Concentration, Temperature and with Different Ionic Content: A Study Using UPLC-MS. Food Nutr. Sci. 2011, 2, 1114–1120. [Google Scholar] [CrossRef] [Green Version]
- Fantini, M.; Benvenuto, M.; Masuelli, L.; Frajese, G.V.; Tresoldi, I.; Modesti, A.; Bei, R. In Vitro and in Vivo Antitumoral Effects of Combinations of Polyphenols, or Polyphenols and Anticancer Drugs: Perspectives on Cancer Treatment. Int. J. Mol. Sci. 2015, 16, 9236–9282. [Google Scholar] [CrossRef] [Green Version]
- Jadid, M.F.S.; Shademan, B.; Chavoshi, R.; Seyyedsani, N.; Aghaei, E.; Taheri, E.; Goleij, P.; Hajazimian, S.; Karamad, V.; Behroozi, J.; et al. Enhanced anticancer potency of hydroxytyrosol and curcumin by PLGA-PAA nano-encapsulation on PANC-1 pancreatic cancer cell line. Environ. Toxicol. 2021, 36, 1043–1051. [Google Scholar] [CrossRef]
- Annunziata, G.; Jiménez-García, M.; Capó, X.; Moranta, D.; Arnone, A.; Tenore, G.C.; Sureda, A.; Tejada, S. Microencapsulation as a tool to counteract the typical low bioavailability of polyphenols in the management of diabetes. Food Chem. Toxicol. 2020, 139, 111248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; McClements, D.J.; Wei, Z.; Wang, G.; Liu, X.; Liu, F. Delivery of synergistic polyphenol combinations using biopolymer-based systems: Advances in physicochemical properties, stability and bioavailability. Crit. Rev. Food Sci. Nutr. 2020, 60, 2083–2097. [Google Scholar] [CrossRef] [PubMed]
- De Leo, V.; Milano, F.; Mancini, E.; Comparelli, R.; Giotta, L.; Nacci, A.; Longobardi, F.; Garbetta, A.; Agostiano, A.; Catucci, L. Encapsulation of Curcumin-Loaded Liposomes for Colonic Drug Delivery in a pH-Responsive Polymer Cluster Using a pH-Driven and Organic Solvent-Free Process. Molecules 2018, 23, 739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Leo, V.; Di Gioia, S.; Milano, F.; Fini, P.; Comparelli, R.; Mancini, E.; Agostiano, A.; Conese, M.; Catucci, L. Eudragit S100 Entrapped Liposome for Curcumin Delivery: Anti-Oxidative Effect in Caco-2 Cells. Coatings 2020, 10, 114. [Google Scholar] [CrossRef] [Green Version]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Kaminaga, Y.; Nagatsu, A.; Akiyama, T.; Sugimoto, N.; Yamazaki, T.; Maitani, T.; Mizukami, H. Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthus roseus. FEBS Lett. 2003, 555, 311–316. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, F.; Iacovino, S.; Sacco, P.; De Leonardis, A.; Ceglie, A.; Lopez, F. Progress in Colloid Delivery Systems for Protection and Delivery of Phenolic Bioactive Compounds: Two Study Cases—Hydroxytyrosol and Curcumin. Molecules 2022, 27, 921. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.; Costa, M.; Ferreira, M.; Gameiro, P.; Paiva-Martins, F. A new family of hydroxytyrosol phenolipids for the antioxidant protection of liposomal systems. Biochim. Biophys. Acta (BBA)—Biomembr. 2021, 1863, 183505. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.O.; Laszlo, J.A.; Compton, D.L. Hydroxytyrosol and tyrosol esters partitioning into, location within, and effect on DOPC liposome bilayer behavior. Biochim. Biophys. Acta (BBA)—Biomembr. 2015, 1848, 1175–1182. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.-J.; Qin, F.G.F.; Tu, J.-L.; Li, B. Preparation, Characterization, and Antioxidant Activity Evaluation of Liposomes Containing Water-Soluble Hydroxytyrosol from Olive. Molecules 2017, 22, 870. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Caro, G.; Sarriá, B.; Madrona, A.; Espartero, J.L.; Escuderos, M.E.; Bravo, L.; Mateos, R. Digestive stability of hydroxytyrosol, hydroxytyrosyl acetate and alkyl hydroxytyrosyl ethers. Int. J. Food Sci. Nutr. 2012, 63, 703–707. [Google Scholar] [CrossRef] [Green Version]
- De Leo, V.; Milano, F.; Agostiano, A.; Catucci, L. Recent Advancements in Polymer/Liposome Assembly for Drug Delivery: From Surface Modifications to Hybrid Vesicles. Polymers 2021, 13, 1027. [Google Scholar] [CrossRef]
- De Leo, V.; Maurelli, A.M.; Giotta, L.; Catucci, L. Liposomes containing nanoparticles: Preparation and applications. Colloids Surfaces. B Biointerfaces 2022, 218, 112737. [Google Scholar] [CrossRef]
- Tangorra, R.R.; Operamolla, A.; Milano, F.; Hassan Omar, O.; Henrard, J.; Comparelli, R.; Italiano, F.; Agostiano, A.; De Leo, V.; Marotta, R.; et al. Assembly of a photosynthetic reaction center with ABA tri-block polymersomes: Highlights on protein localization. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2015, 14, 1844–1852. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B 2019, 9, 36–48. [Google Scholar] [CrossRef]
- Liu, W.; Ye, A.; Liu, W.; Liu, C.; Han, J.; Singh, H. Behaviour of liposomes loaded with bovine serum albumin during in vitro digestion. Food Chem. 2015, 175, 16–24. [Google Scholar] [CrossRef]
- Niu, M.; Lu, Y.; Hovgaard, L.; Guan, P.; Tan, Y.; Lian, R.; Qi, J.; Wu, W. Hypoglycemic activity and oral bioavailability of insulin-loaded liposomes containing bile salts in rats: The effect of cholate type, particle size and administered dose. Eur. J. Pharm. Biopharm. 2012, 81, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Minato, S.; Iwanaga, K.; Kakemi, M.; Yamashita, S.; Oku, N. Application of polyethyleneglycol (PEG)-modified liposomes for oral vaccine: Effect of lipid dose on systemic and mucosal immunity. J. Control. Release 2003, 89, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, K.; Ono, S.; Narioka, K.; Morimoto, K.; Kakemi, M.; Yamashita, S.; Nango, M.; Oku, N. Oral delivery of insulin by using surface coating liposomes: Improvement of stability of insulin in GI tract. Int. J. Pharm. 1997, 157, 73–80. [Google Scholar] [CrossRef]
- De Leo, V.; Ruscigno, S.; Trapani, A.; Di Gioia, S.; Milano, F.; Mandracchia, D.; Comparelli, R.; Castellani, S.; Agostiano, A.; Trapani, G.; et al. Preparation of drug-loaded small unilamellar liposomes and evaluation of their potential for the treatment of chronic respiratory diseases. Int. J. Pharm. 2018, 545, 378–388. [Google Scholar] [CrossRef]
- Long, F.; Yang, H.; Xu, Y.; Hao, H.; Li, P. A strategy for the identification of combinatorial bioactive compounds contributing to the holistic effect of herbal medicines. Sci. Rep. 2015, 5, 12361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rietjens, S.J.; Bast, A.; Vente, J.d.; Haenen, G.R.M.M. The olive oil antioxidant hydroxytyrosol efficiently protects against the oxidative stress-induced impairment of the NO• response of isolated rat aorta. Am. J. Physiol.-Heart Circ. Physiol. 2007, 292, H1931–H1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Ye, A.; Liu, C.; Liu, W.; Singh, H. Structure and integrity of liposomes prepared from milk- or soybean-derived phospholipids during in vitro digestion. Food Res. Int. 2012, 48, 499–506. [Google Scholar] [CrossRef]
- Liu, W.; Hou, Y.; Jin, Y.; Wang, Y.; Xu, X.; Han, J. Research progress on liposomes: Application in food, digestion behavior and absorption mechanism. Trends Food Sci. Technol. 2020, 104, 177–189. [Google Scholar] [CrossRef]
- Carrière, F.; Moreau, H.; Raphel, V.; Laugier, R.; Benicourt, C.; Junien, J.-L.; Verger, R. Purification and biochemical characterization of dog gastric lipase. Eur. J. Biochem. 1991, 202, 75–83. [Google Scholar] [CrossRef]
- Liu, W.; Ye, A.; Han, F.; Han, J. Advances and challenges in liposome digestion: Surface interaction, biological fate, and GIT modeling. Adv. Colloid Interface Sci. 2019, 263, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.; Singh, A.; Kumar, R.V.; Singh, S.; Kumria, R.; Bhinge, J. Oral colon-specific drug delivery of protein and peptide drugs. Crit. Rev. Ther. Drug Carr. Syst. 2007, 24, 63–92. [Google Scholar] [CrossRef] [PubMed]
- Maherani, B.; Arab-Tehrany, E.; Kheirolomoom, A.; Geny, D.; Linder, M. Calcein release behavior from liposomal bilayer; influence of physicochemical/mechanical/structural properties of lipids. Biochimie 2013, 95, 2018–2033. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Pan, M.-H.; Cheng, A.-L.; Lin, L.-I.; Ho, Y.-S.; Hsieh, C.-Y.; Lin, J.-K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Atzeri, A.; Lucas, R.; Incani, A.; Peñalver, P.; Zafra-Gómez, A.; Melis, M.P.; Pizzala, R.; Morales, J.C.; Deiana, M. Hydroxytyrosol and tyrosol sulfate metabolites protect against the oxidized cholesterol pro-oxidant effect in Caco-2 human enterocyte-like cells. Food Function. 2016, 7, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ramiro, I.; Martín, M.Á.; Ramos, S.; Bravo, L.; Goya, L. Olive oil hydroxytyrosol reduces toxicity evoked by acrylamide in human Caco-2 cells by preventing oxidative stress. Toxicology 2011, 288, 43–48. [Google Scholar] [CrossRef] [Green Version]
- De Leo, V.; Mattioli-Belmonte, M.; Cimmarusti, M.T.; Panniello, A.; Dicarlo, M.; Milano, F.; Agostiano, A.; De Giglio, E.; Catucci, L. Liposome-modified titanium surface: A strategy to locally deliver bioactive molecules. Colloids Surf. B Biointerfaces 2017, 158, 387–396. [Google Scholar] [CrossRef]
- Milano, F.; Ciriaco, F.; Trotta, M.; Chirizzi, D.; De Leo, V.; Agostiano, A.; Valli, L.; Giotta, L.; Guascito, M.R. Design and modelling of a photo-electrochemical transduction system based on solubilized photosynthetic reaction centres. Electrochim. Acta 2019, 293, 105–115. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Dziki, D.; Baraniak, B.; Lin, R. The effect of simulated digestion in vitro on bioactivity of wheat bread with Tartary buckwheat flavones addition. LWT—Food Sci. Technol. 2009, 42, 137–143. [Google Scholar] [CrossRef]
- Mastrogiacomo, D.; Lenucci, M.S.; Bonfrate, V.; Di Carolo, M.; Piro, G.; Valli, L.; Rescio, L.; Milano, F.; Comparelli, R.; De Leo, V.; et al. Lipid/detergent mixed micelles as a tool for transferring antioxidant power from hydrophobic natural extracts into bio-deliverable liposome carriers: The case of lycopene rich oleoresins. RSC Adv. 2015, 5, 3081–3093. [Google Scholar] [CrossRef]
- Di Gioia, S.; Trapani, A.; Cassano, R.; Di Gioia, M.L.; Trombino, S.; Cellamare, S.; Bolognino, I.; Hossain, M.N.; Sanna, E.; Trapani, G.; et al. Nose-to-brain delivery: A comparative study between carboxymethyl chitosan based conjugates of dopamine. Int. J. Pharm. 2021, 599, 120453. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Panda, A.K.; Majumdar, D.K. Eudragit S100 entrapped insulin microspheres for oral delivery. AAPS PharmSciTech 2005, 6, E100–E107. [Google Scholar] [CrossRef] [PubMed]








| Sample | Coating | Cur 1 | HT 1 | Cur + HT 1 | |||
|---|---|---|---|---|---|---|---|
| Diameter (nm) | PDI | Diameter (nm) | PDI | Diameter (nm) | PDI | ||
| Type 1 | none | 115.5 ± 0.6 | 0.133 ± 0.009 | 109.6 ± 1.3 | 0.127 ± 0.011 | 114.5 ± 0.4 | 0.123 ± 0.010 |
| Type 2 | PEG-2000 | 100.6 ± 0.7 | 0.114 ± 0.012 | 108.7 ± 0.5 | 0.122 ± 0.014 | 102.6 ± 0.6 | 0.116 ± 0.008 |
| Type 3, pH < 7 | PEG-2000/ EuS100 | >1000 | N.D. | >1000 | N.D. | >1000 | N.D. |
| Type 3, pH ≥ 7 | PEG-2000/ EuS100 | 114.3 ± 0.8 | 0.22 ± 0.02 | 110 ± 2 | 0.213 ± 0.018 | 113 ± 4 | 0.23 ± 0.03 |
| Sample | Cur 1 | HT 1 | Cur + HT 1 | |||||
|---|---|---|---|---|---|---|---|---|
| EE% | LC% | EE% | LC% | Cur-EE% | Cur-LC% | HT-EE% | HT-LC% | |
| Type 1 | 81 ± 2 | 0.30 ± 0.02 | 2.5 ± 1.0 | 2.1 ± 0.9 | 69 ± 7 | 0.25 ± 0.02 | 1.9 ± 0.5 | 1.7 ± 0.3 |
| Type 2 | 87 ± 3 | 0.32 ± 0.04 | 2.0 ± 1.1 | 1.6 ± 0.9 | 70 ± 9 | 0.26 ± 0.03 | 2.0 ± 0.8 | 1.7 ± 0.7 |
| Type 3 | 87 ± 3 | 0.32 ± 0.04 2 | 1.7 ± 0.9 | 1.4 ± 1.2 2 | 70 ± 9 | 0.26 ± 0.03 2 | 1.7 ± 0.6 | 1.5 ± 0.5 2 |
| Sample | Diameter (nm) | PDI | ζ-potential (mV) | |
|---|---|---|---|---|
| Type 1 | As prepared | 114.5 ± 0.4 | 0.123 ± 0.010 | −1.7 ± 0.3 |
| After mouth digestion | 114.9 ± 1.8 | 0.112 ± 0.005 | −4.8 ± 0.2 | |
| After gastric digestion | 109.6 ± 1.3 | 0.15 ± 0.03 | +9.7 ± 1.3 | |
| After intestinal digestion | 119.0 ± 1.0 | 0.156 ± 0.008 | −19.6 ± 0.8 | |
| Type 2 | As prepared | 102.6 ± 0.6 | 0.116 ± 0.008 | −9.3 ± 0.3 |
| After mouth digestion | 108 ± 2 | 0.18 ± 0.03 | −5.2 ± 0.9 | |
| After gastric digestion | 109.7 ± 0.5 | 0.18 ± 0.03 | −0.6 ± 0.2 | |
| After intestinal digestion | 110 ± 2 | 0.20 ± 0.03 | −14.2 ± 1.5 | |
| Type 3 | As prepared | >1000 | - | −20.9 ± 1.3 |
| After mouth digestion | >1000 | - | −16.4 ± 2 | |
| After gastric digestion | >1000 | - | +0.8 ± 0.4 | |
| After intestinal digestion | 124 ± 3 | 0.23 ± 0.02 | −25.3 ± 0.2 |
| Sample | Release in Mouth Phase | Release in Gastric Phase | Release in Intestinal Phase |
|---|---|---|---|
| Type 1 | (17.9 ± 1.5)% | (16 ± 2)% | (54 ± 4)% |
| Type 2 | (12 ± 2)% | (9.5 ± 1.4)% | (36 ± 2)% |
| Type 3 | (0.7 ± 0.2)% | (0.040 ± 0.007)% | (35 ± 3)% |
| Sample | Cur (μM) | HT (μM) | TEAC (Absolute) | TEAC (μeq T/mL Liposome Suspension) |
|---|---|---|---|---|
| Empty liposomes | 0 | 0 | -- | 0.011 ± 0.003 |
| HT-liposomes | 0 | 280 | 0.90 ± 0.02 | 0.25 ± 0.06 |
| Free HT 1 | 0 | 280 | 1.2 ± 0.2 | |
| Cur-liposomes | 36 | 0 | 3.19 ± 0.13 | 0.12 ± 0.05 |
| Free Cur 2 | 27 | 0 | 3.25 ± 0.15 | |
| HT/Cur-liposomes | 32 | 229 | -- | 0.29 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Leo, V.; Maurelli, A.M.; Giotta, L.; Daniello, V.; Di Gioia, S.; Conese, M.; Ingrosso, C.; Ciriaco, F.; Catucci, L. Polymer Encapsulated Liposomes for Oral Co-Delivery of Curcumin and Hydroxytyrosol. Int. J. Mol. Sci. 2023, 24, 790. https://doi.org/10.3390/ijms24010790
De Leo V, Maurelli AM, Giotta L, Daniello V, Di Gioia S, Conese M, Ingrosso C, Ciriaco F, Catucci L. Polymer Encapsulated Liposomes for Oral Co-Delivery of Curcumin and Hydroxytyrosol. International Journal of Molecular Sciences. 2023; 24(1):790. https://doi.org/10.3390/ijms24010790
Chicago/Turabian StyleDe Leo, Vincenzo, Anna Maria Maurelli, Livia Giotta, Valeria Daniello, Sante Di Gioia, Massimo Conese, Chiara Ingrosso, Fulvio Ciriaco, and Lucia Catucci. 2023. "Polymer Encapsulated Liposomes for Oral Co-Delivery of Curcumin and Hydroxytyrosol" International Journal of Molecular Sciences 24, no. 1: 790. https://doi.org/10.3390/ijms24010790