Tobramycin Nanoantibiotics and Their Advantages: A Minireview
Abstract
:1. Introduction
2. Tobramycin
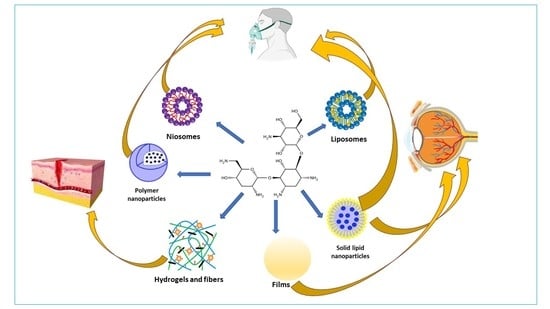
3. Nanosized Carriers for Tobramycin
3.1. Liposomes
3.2. Polymer Nanoparticles
3.3. Solid Lipid Nanoparticles (SLNs)
4. Other Carriers
5. Discussion
6. Conclusions
7. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization, European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe: 2022: 2020 Data; Publications Office of the European Union: Luxembourg, 2022; ISBN 978-92-9498-552-1. [Google Scholar]
- Lei, R.; Hou, J.; Chen, Q.; Yuan, W.; Cheng, B.; Sun, Y.; Jin, Y.; Ge, L.; Ben-Sasson, S.A.; Chen, J.; et al. Self-Assembling Myristoylated Human α-Defensin 5 as a Next-Generation Nanobiotics Potentiates Therapeutic Efficacy in Bacterial Infection. ACS Nano 2018, 12, 5284–5296. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
- Berini, F.; Orlandi, V.; Gornati, R.; Bernardini, G.; Marinelli, F. Nanoantibiotics to fight multidrug resistant infections by Gram-positive bacteria: Hope or reality? Biotechnol. Adv. 2022, 57, 107948. [Google Scholar] [CrossRef]
- Sriram, A.; Kalanxhi, E.; Kapoor, G.; Craig, J.; Balasubramanian, R.; Brar, S.; Criscuolo, N.; Hamilton, A.; Klein, E.; Tseng, K.; et al. State of the World’s Antibiotics 2021: A Global Analysis of Antimicrobial Resistance and Its Drivers; Economics & Policy: Washington, DC, USA, 2021. [Google Scholar]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [Green Version]
- Troisi, M.; Andreano, E.; Sala, C.; Kabanova, A.; Rappuoli, R. Vaccines as remedy for antimicrobial resistance and emerging infections. Curr. Opin. Immunol. 2020, 65, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Zurawski, D.V.; McLendon, M.K. Monoclonal Antibodies as an Antibacterial Approach Against Bacterial Pathogens. Antibiotics 2020, 9, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfalzgraff, A.; Brandenburg, K.; Weindl, G. Antimicrobial Peptides and Their Therapeutic Potential for Bacterial Skin Infections and Wounds. Front. Pharmacol. 2018, 9, 281. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Mullish, B.H.; Kelly, C.; Fischer, M. The evolution of the use of faecal microbiota transplantation and emerging therapeutic indications. Lancet 2019, 394, 420–431. [Google Scholar] [CrossRef]
- Rao, K.; Young, V.B. Fecal microbiota transplantation for the management of Clostridium difficile infection. Infect. Dis. Clin. N. Am. 2015, 29, 109–122. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.I.; Maguire, F.; Tsang, K.K.; Gouliouris, T.; Peacock, S.J.; McAllister, T.A.; McArthur, A.G.; Beiko, R.G. Machine Learning for Antimicrobial Resistance Prediction: Current Practice, Limitations, and Clinical Perspective. Clin. Microbiol. Rev. 2022, 35, e0017921. [Google Scholar] [CrossRef]
- Chahine, E.B.; Dougherty, J.A.; Thornby, K.A.; Guirguis, E.H. Antibiotic Approvals in the Last Decade: Are We Keeping Up With Resistance? Ann. Pharmacother. 2022, 56, 441–462. [Google Scholar] [CrossRef] [PubMed]
- Elfadil, D.; Elkhatib, W.F.; El-Sayyad, G.S. Promising advances in nanobiotic-based formulations for drug specific targeting against multidrug-resistant microbes and biofilm-associated infections. Microb. Pathog. 2022, 170, 105721. [Google Scholar] [CrossRef] [PubMed]
- Bazán Henostroza, M.A.; Diniz Tavares, G.; Nishitani Yukuyama, M.; De Souza, A.; José Barbosa, E.; Carlos Avino, V.; Dos Santos Neto, E.; Rebello Lourenço, F.; Löbenberg, R.; Araci Bou-Chacra, N. Antibiotic-loaded lipid-based nanocarrier: A promising strategy to overcome bacterial infection. Int. J. Pharm. 2022, 621, 121782. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Kon, E.; Elia, U.; Peer, D. Principles for designing an optimal mRNA lipid nanoparticle vaccine. Curr. Opin. Biotechnol. 2022, 73, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Liposome as a delivery system for the treatment of biofilm-mediated infections. J. Appl. Microbiol. 2021, 131, 2626–2639. [Google Scholar] [CrossRef]
- Omolo, C.A.; Kalhapure, R.S.; Agrawal, N.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Govender, T. A hybrid of mPEG-b-PCL and G1-PEA dendrimer for enhancing delivery of antibiotics. J. Control. Release 2018, 290, 112–128. [Google Scholar] [CrossRef]
- Forna, N.; Damir, D.; Duceac, L.D.; Dabija, M.G.; Calin, G.; Ichim, D.L.; Gutu, C.; Grierosu, C.; Eva, L.; Ciuhodaru, M.I.; et al. Nano-Architectonics of Antibiotic-Loaded Polymer Particles as Vehicles for Active Molecules. Appl. Sci. 2022, 12, 1998. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, Y.; Shen, A.; Yang, Y.; Diao, L.; Wang, L.; Cai, D.; Hu, Y. Injectable Chitosan-Based Thermosensitive Hydrogel/Nanoparticle-Loaded System for Local Delivery of Vancomycin in the Treatment of Osteomyelitis. Int. J. Nanomed. 2020, 15, 5855–5871. [Google Scholar] [CrossRef]
- Scutera, S.; Argenziano, M.; Sparti, R.; Bessone, F.; Bianco, G.; Bastiancich, C.; Castagnoli, C.; Stella, M.; Musso, T.; Cavalli, R. Enhanced Antimicrobial and Antibiofilm Effect of New Colistin-Loaded Human Albumin Nanoparticles. Antibiotics 2021, 10, 57. [Google Scholar] [CrossRef]
- Serio, A.W.; Keepers, T.; Andrews, L.; Krause, K.M. Aminoglycoside Revival: Review of a Historically Important Class of Antimicrobials Undergoing Rejuvenation. EcoSal Plus 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyhanoglu, G.; Reddivari, A.K.R. Tobramycin. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Beaulac, C.; Sachetelli, S.; Lagacé, J. Aerosolization of low phase transition temperature liposomal tobramycin as a dry powder in an animal model of chronic pulmonary infection caused by Pseudomonas aeruginosa. J. Drug. Target 1999, 7, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Smyth, A.R.; Bell, S.C.; Bojcin, S.; Bryon, M.; Duff, A.; Flume, P.; Kashirskaya, N.; Munck, A.; Ratjen, F.; Schwarzenberg, S.J.; et al. European Cystic Fibrosis Society Standards of Care: Best Practice guidelines. J. Cyst. Fibros. 2014, 13 (Suppl. S1), S23–S42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waters, V.; Smyth, A. Cystic fibrosis microbiology: Advances in antimicrobial therapy. J. Cyst. Fibros. 2015, 14, 551–560. [Google Scholar] [CrossRef]
- Marier, J.F.; Lavigne, J.; Ducharme, M.P. Pharmacokinetics and efficacies of liposomal and conventional formulations of tobramycin after intratracheal administration in rats with pulmonary Burkholderia cepacia infection. Antimicrob. Agents Chemother. 2002, 46, 3776–3781. [Google Scholar] [CrossRef] [Green Version]
- Marier, J.F.; Brazier, J.L.; Lavigne, J.; Ducharme, M.P. Liposomal tobramycin against pulmonary infections of Pseudomonas aeruginosa: A pharmacokinetic and efficacy study following single and multiple intratracheal administrations in rats. J. Antimicrob. Chemother. 2003, 52, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Halwani, M.; Blomme, S.; Suntres, Z.E.; Alipour, M.; Azghani, A.O.; Kumar, A.; Omri, A. Liposomal bismuth-ethanedithiol formulation enhances antimicrobial activity of tobramycin. Int. J. Pharm. 2008, 358, 278–284. [Google Scholar] [CrossRef]
- Mahdiun, F.; Mansouri, S.; Khazaeli, P.; Mirzaei, R. The effect of tobramycin incorporated with bismuth-ethanedithiol loaded on niosomes on the quorum sensing and biofilm formation of Pseudomonas aeruginosa. Microb. Pathog 2017, 107, 129–135. [Google Scholar] [CrossRef]
- Alzahrani, N.M.; Booq, R.Y.; Aldossary, A.M.; Bakr, A.A.; Almughem, F.A.; Alfahad, A.J.; Alsharif, W.K.; Jarallah, S.J.; Alharbi, W.S.; Alsudir, S.A.; et al. Liposome-Encapsulated Tobramycin and IDR-1018 Peptide Mediated Biofilm Disruption and Enhanced Antimicrobial Activity against Pseudomonas aeruginosa. Pharmaceutics 2022, 14, 960. [Google Scholar] [CrossRef]
- Alarfaj, R.E.; Alkhulaifi, M.M.; Al-Fahad, A.J.; Aljihani, S.; Yassin, A.E.B.; Alghoribi, M.F.; Halwani, M.A. Antibacterial Efficacy of Liposomal Formulations Containing Tobramycin and N-Acetylcysteine against Tobramycin-Resistant Escherichia coli, Klebsiella pneumoniae, and Acinetobacter baumannii. Pharmaceutics 2022, 14, 130. [Google Scholar] [CrossRef]
- Hill, M.; Cunningham, R.N.; Hathout, R.M.; Johnston, C.; Hardy, J.G.; Migaud, M.E. Formulation of Antimicrobial Tobramycin Loaded PLGA Nanoparticles via Complexation with AOT. J. Funct. Biomater. 2019, 10, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deacon, J.; Abdelghany, S.M.; Quinn, D.J.; Schmid, D.; Megaw, J.; Donnelly, R.F.; Jones, D.S.; Kissenpfennig, A.; Elborn, J.S.; Gilmore, B.F.; et al. Antimicrobial efficacy of tobramycin polymeric nanoparticles for Pseudomonas aeruginosa infections in cystic fibrosis: Formulation, characterisation and functionalisation with dornase alfa (DNase). J. Control. Release 2015, 198, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Sastre, M.; Pastor, M.; Esquisabel, A.; Sans, E.; Viñas, M.; Fleischer, A.; Palomino, E.; Bachiller, D.; Pedraz, J.L. Pulmonary delivery of tobramycin-loaded nanostructured lipid carriers for Pseudomonas aeruginosa infections associated with cystic fibrosis. Int. J. Pharm. 2016, 498, 263–273. [Google Scholar] [CrossRef]
- Chetoni, P.; Burgalassi, S.; Monti, D.; Tampucci, S.; Tullio, V.; Cuffini, A.M.; Muntoni, E.; Spagnolo, R.; Zara, G.P.; Cavalli, R. Solid lipid nanoparticles as promising tool for intraocular tobramycin delivery: Pharmacokinetic studies on rabbits. Eur. J. Pharm. Biopharm. 2016, 109, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Messiaen, A.S.; Forier, K.; Nelis, H.; Braeckmans, K.; Coenye, T. Transport of nanoparticles and tobramycin-loaded liposomes in Burkholderia cepacia complex biofilms. PLoS ONE 2013, 8, e79220. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Cabra, N.; Movellan, J.; Marradi, M.; Gracia, R.; Salvador, C.; Dupin, D.; Loinaz, I.; Torrents, E. Neutralization of ionic interactions by dextran-based single-chain nanoparticles improves tobramycin diffusion into a mature biofilm. NPJ Biofilms Microbiomes 2022, 8, 52. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beitelshees, M.; Hill, A.; Jones, C.H.; Pfeifer, B.A. Phenotypic Variation during Biofilm Formation: Implications for Anti-Biofilm Therapeutic Design. Materials 2018, 11, 1086. [Google Scholar] [CrossRef] [Green Version]
- Meers, P.; Neville, M.; Malinin, V.; Scotto, A.W.; Sardaryan, G.; Kurumunda, R.; Mackinson, C.; James, G.; Fisher, S.; Perkins, W.R. Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J. Antimicrob. Chemother. 2008, 61, 859–868. [Google Scholar] [CrossRef]
- Dos Santos Ramos, M.A.; Da Silva, P.B.; Spósito, L.; De Toledo, L.G.; Bonifácio, B.V.; Rodero, C.F.; Dos Santos, K.C.; Chorilli, M.; Bauab, T.M. Nanotechnology-based drug delivery systems for control of microbial biofilms: A review. Int. J. Nanomed. 2018, 13, 1179–1213. [Google Scholar] [CrossRef]
- Wu, C.L.; Domenico, P.; Hassett, D.J.; Beveridge, T.J.; Hauser, A.R.; Kazzaz, J.A. Subinhibitory bismuth-thiols reduce virulence of Pseudomonas aeruginosa. Am. J. Respir. Cell Mol. Biol. 2002, 26, 731–738. [Google Scholar] [CrossRef] [PubMed]
- de Kievit, T.R. Quorum sensing in Pseudomonas aeruginosa biofilms. Environ. Microbiol. 2009, 11, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, F.; d’Angelo, I.; Coletta, C.; d’Emmanuele di Villa Bianca, R.; Sorrentino, R.; Perfetto, B.; Tufano, M.A.; Miro, A.; La Rotonda, M.I.; Quaglia, F. Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: Modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J. Control. Release 2012, 157, 149–159. [Google Scholar] [CrossRef]
- Shirodkar, R.K.; Kumar, L.; Mutalik, S.; Lewis, S.A. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Emerging Lipid Based Drug Delivery Systems. Pharm. Chem. J. 2019, 53, 440–453. [Google Scholar] [CrossRef]
- Madkhali, O.A. Perspectives and Prospective on Solid Lipid Nanoparticles as Drug Delivery Systems. Molecules 2022, 27, 1543. [Google Scholar] [CrossRef]
- Cavalli, R.; Gasco, M.R.; Chetoni, P.; Burgalassi, S.; Saettone, M.F. Solid lipid nanoparticles (SLN) as ocular delivery system for tobramycin. Int. J. Pharm. 2002, 238, 241–245. [Google Scholar] [CrossRef]
- Liang, L.; Liu, T.; Ouyang, Q.; Li, S.; Li, C. Solid phase synthesis of oxidized sodium alginate-tobramycin conjugate and its application for infected wound healing. Carbohydr. Polym. 2022, 295, 119843. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mu, L.; Zhao, X.; Han, Y.; Guo, B. Bacterial Growth-Induced Tobramycin Smart Release Self-Healing Hydrogel for Pseudomonas aeruginosa-Infected Burn Wound Healing. ACS Nano 2022, 16, 13022–13036. [Google Scholar] [CrossRef]
- Xu, K.; Shan, W.; Hu, N.; Wang, J.; Zhou, W.; Müller-Buschbaum, P.; Zhong, Q. High efficiency of in-situ cross-linking and acid triggered drug delivery by introducing tobramycin into injectable and biodegradable hydrogels. Colloids Surf B Biointerfaces 2022, 218, 112756. [Google Scholar] [CrossRef]
- Boffoli, D.; Bellato, F.; Avancini, G.; Gurnani, P.; Yilmaz, G.; Romero, M.; Robertson, S.; Moret, F.; Sandrelli, F.; Caliceti, P.; et al. Tobramycin-loaded complexes to prevent and disrupt Pseudomonas aeruginosa biofilms. Drug. Deliv. Transl. Res. 2022, 12, 1788–1810. [Google Scholar] [CrossRef]
- Li, Z.; Mei, S.; Dong, Y.; She, F.; Li, C.; Li, Y.; Kong, L. Oxidized Chitosan-Tobramycin (OCS-TOB) Submicro-Fibers for Biomedical Applications. Pharmaceutics 2022, 14, 1197. [Google Scholar] [CrossRef] [PubMed]
- Rosalia, M.; Ravipati, P.; Grisoli, P.; Dorati, R.; Genta, I.; Chiesa, E.; Bruni, G.; Conti, B. Tobramycin Supplemented Small-Diameter Vascular Grafts for Local Antibiotic Delivery: A Preliminary Formulation Study. Int. J. Mol. Sci. 2021, 22, 13557. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ren, L.; Long, K.; Wang, L.; Wang, Y. Preparation and characterization of a novel tobramycin-containing antibacterial collagen film for corneal tissue engineering. Acta Biomater. 2014, 10, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.K.; Dreifus, J.E.; Reichhardt, C.; Storek, K.M.; Secor, P.R.; Wozniak, D.J.; Hisert, K.B.; Parsek, M.R. Pseudomonas aeruginosa aggregates in cystic fibrosis sputum produce exopolysaccharides that likely impede current therapies. Cell Rep. 2021, 34, 108782. [Google Scholar] [CrossRef]
- Domalaon, R.; Idowu, T.; Zhanel, G.G.; Schweizer, F. Antibiotic Hybrids: The Next Generation of Agents and Adjuvants against Gram-Negative Pathogens? Clin. Microbiol. Rev. 2018, 31, e00077-17. [Google Scholar] [CrossRef]




| Nanocarrier Type /Admin. Route | Composition and Encapsulation Efficiency (EE) | Encapsulated Tobramycin Performance (Compared to Free Drug) | Refs. |
|---|---|---|---|
| Liposomes/aerosol | FluidosomesTM | CFU lowering of about 300 time against isolate of P. aeruginosa, PA 508. | [25] |
| Liposomes/aerosol | 10:1 molar ratio dipalmitoylphosphatidylcholine (DPPC): dimyristoylphosphatidylglycerol (DMPG) | Significant increase in t1/2_ from the lungs against BC. ** | [28] |
| Liposomes/intratracheal | 10:1 molar ratio DPPC:DMPG | Significant increase in t1/2_ from the lungs against isolate of PA *, PA 508. | [29] |
| Liposomes | 2:1 molar ratio Disteroylphospahtidylcholine (DSPC): cholesterol, bismuth-ethanedithiol | MIC reduction by 3 to 8-fold against PA and BC, respectively. | [30] |
| Niosomes | Span 40, Tween 40, cholesterol, ratio 70:30 | A 4-fold MIC reduction against clinical strains of PA ATCC 27583. A total of 80% inhibition of biofilm formation. Inhibition of N-Acyl homoserine lactones (AHL) production. | [31] |
| Liposomes | 3_-[N-(N’, N’-dimethyl aminoethane)-carbamoyl], cholesterol hydrochloride (DC-Chol), 1, 2-dioleoyl-snglycero-3-phosphoethanolamine (DOPE). Anti-biofilm peptide (IDR 1018) | Significant increase in antibiofilm activity against PA and its biofilm, except for IDR1018 addition. | [32] |
| Liposomes | 1,2-Dimyristoyl-sn-glycero-3-phosphoethanolamine (DMPE), DPPC, cholesterol. N-acetylcysteine | Significant increase in antimicrobial and antibiofilm activity gainst E. coli, K. pneumoniae, and A. baumannii. | [33] |
| Polymer nanoparticles / in vitro | Polylactide-co-glycolide (PLGA) Resomer RG503, PLGA RG 502H, dioctylsulfosuccinate (AOT). 95% encaps efficiency (EE). | MIC kept same values of not encapsulated drug against PA. | [34] |
| Polymer nanoparticles/ in vitro | Sodium alginate, chitosan (low MW). Dornase alfa (DNase). 45% EE | Slight significant improvement of DNase tobramycin NPs antimicrobial and antibiofilm activities against PA. | [35] |
| SLN/ in vitro and intratrachea to mice | Glycerol distearate, type I, glyceryl dibehenate, Poloxamer 188. 93–99% EE | Sustained drug release into lungs. | [36] |
| SLN/intraocular to rabbits | Stearic acid, soya phosphatidylcholine, soya tauroglycolate. 95% EE. 2.5% drug loading | A total 3-fold bioavailability increase. | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosalia, M.; Chiesa, E.; Tottoli, E.M.; Dorati, R.; Genta, I.; Conti, B.; Pisani, S. Tobramycin Nanoantibiotics and Their Advantages: A Minireview. Int. J. Mol. Sci. 2022, 23, 14080. https://doi.org/10.3390/ijms232214080
Rosalia M, Chiesa E, Tottoli EM, Dorati R, Genta I, Conti B, Pisani S. Tobramycin Nanoantibiotics and Their Advantages: A Minireview. International Journal of Molecular Sciences. 2022; 23(22):14080. https://doi.org/10.3390/ijms232214080
Chicago/Turabian StyleRosalia, Mariella, Enrica Chiesa, Erika Maria Tottoli, Rossella Dorati, Ida Genta, Bice Conti, and Silvia Pisani. 2022. "Tobramycin Nanoantibiotics and Their Advantages: A Minireview" International Journal of Molecular Sciences 23, no. 22: 14080. https://doi.org/10.3390/ijms232214080