Medium Bandgap Polymers for Efficient Non-Fullerene Polymer Solar Cells—An In-Depth Study of Structural Diversity of Polymer Structure
Abstract
:1. Introduction
2. Results and Discussion
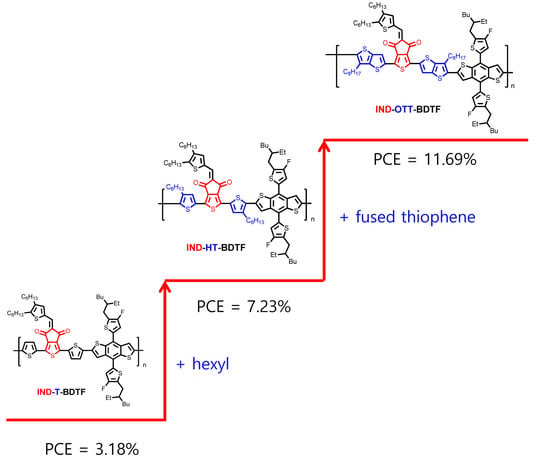
2.1. Synthesis and Characterization
2.2. Optical and Electrochemical Behaviors
2.3. Photovoltaic Property
2.4. Morphology Study
3. Materials and Methods
3.1. Materials and Instruments
3.2. Synthesis of Monomers and Polymers
3.2.1. Synthesis of 1,3-Dibromo-4H-cyclopenta[c]thiophene-4,6(5H)-dione (IND)
3.2.2. Synthesis of 2-Bromo-3-Hexylthiophene (2)
3.2.3. Synthesis of 2,3-Dihexylthiophene (3)
3.2.4. Synthesis of 4,5-Dihexylthiophene-2-Carbaldehyde (4)
3.2.5. Synthesis of 1,3-Dibromo-5((4,5-dihexylthiophen-2-yl)methylene)4Hcyclopenta[c]thiophene-4,6(5H)-dione (5)
3.2.6. Synthesis of 5-((4,5-Dihexylthiophen-2-yl)methylene)-1,3-di(thiophen-2-yl)-4H-cyclopenta[c]thiophene-4,6(5H)-dione (6)
3.2.7. Synthesis of 1,3-Bis(5-bromothiophen-2-yl)-5-((4,5-dihexylthiophen-2-yl)methylene)-4H-cyclopenta[c]thiophene-4,6(5H)-dione (M1)
3.2.8. Synthesis of 5-((4,5-Dihexylthiophen-2-yl)methylene)-1,3-bis(4-hexylthiophen-2-yl)-4H-cyclopenta[c]thiophene-4,6(5H)-dione (7)
3.2.9. Synthesis of 1,3-Bis(5-bromo-4-hexylthiophen-2-yl)-5-((4,5-dihexylthiophen-2-yl)methylene)-4H-cyclopenta[c]thiophene-4,6(5H)-dione (M2)
3.2.10. Synthesis of 5-((4,5-Dihexylthiophen-2-yl)methylene)-1,3-bis(6-octylthieno [3,2-b]thiophen-2-yl)-4H-cyclopenta[c]thiophene-4,6(5H)-dione (8)
3.2.11. Synthesis of 1,3-Bis(5-bromo-6-octylthieno [3,2-b]thiophen-2-yl)-5-((4,5-dihexylthiophen-2-yl)methylene)-4H-cyclopenta[c]thiophene-4,6(5H)-dione (M3)
3.2.12. Synthesis of IND-T-BDTF, IND-HT-BDTF and IND-OTT-BDTF
3.3. Fabrication and Analysis of PSCs
3.4. Fabrication of Hole- and Electron-Only Devices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reddy, S.S.; Aryal, U.K.; Jin, H.; Gokulnath, T.; Rajalapati, D.G.; Kranthiraja, K.; Shin, S.T.; Jin, S.H. A new benzodithiophene based donor-acceptor π-conjugated polymer for organic solar cells. Macromol. Res. 2020, 28, 179–183. [Google Scholar] [CrossRef]
- Yan, C.; Barlow, S.; Wang, Z.; Yan, H.; Jen, A.K.Y.; Marder, S.R.; Zhan, X. Non-fullerene acceptors for organic solar cells. Nat. Rev. Mater. 2018, 3, 18003. [Google Scholar] [CrossRef]
- Zhang, S.; Qin, Y.; Zhu, J.; Hou, J. Over 14% efficiency in polymer solar cells enabled by a chlorinated polymer donor. Adv. Mater. 2018, 30, e1800868. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Feng, K.; Lee, Y.W.; Woo, H.Y.; Zhang, G.; Peng, Q. Subtle polymer donor and molecular acceptor design enable efficient polymer solar cells with a very small energy loss. Adv. Funct. Mater. 2020, 30, 1907570. [Google Scholar] [CrossRef]
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polymer—Fullerene bulk—Heterojunction solar cells. Adv. Mater. 2009, 21, 1323. [Google Scholar] [CrossRef]
- Heeger, A.J. 25th anniversary article: Bulk heterojunction solar cells: Understanding the mechanism of operation. Adv. Mater. 2014, 26, 10–28. [Google Scholar] [CrossRef]
- Nelson, J. Polymer: Fullerene bulk heterojunction solar cells. Mater. Today 2011, 14, 462–470. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.L.; Lau, T.K.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-junction organic solar cell with over 15% efficiency using fused-ring acceptor with electron-deficient core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]
- Nugraha, D.F.; Son, D.H.; Wardani, R.P.; Lee, S.W.; Whang, D.R.; Kim, J.H.; Chang, D.W. Strategic structural evolution for enhancing the photovoltaic performance of quinoxaline-based polymers. J. Ind. Eng. Chem. 2022, 114, 331–337. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Zhang, J.; Zhang, T.; Wang, Y.; Hong, L.; Xian, K.; Xu, B.; Zhang, S.; Peng, J.; et al. Over 16% efficiency organic photovoltaic cells enabled by a chlorinated acceptor with increased open-circuit voltages. Nat. Commun. 2019, 10, 2515. [Google Scholar] [CrossRef]
- Song, J.; Li, C.; Zhu, L.; Guo, J.; Xu, J.; Zhang, X.; Weng, K.; Zhang, K.; Min, J.; Hao, X.; et al. Ternary organic solar cells with efficiency >16.5% based on two compatible nonfullerene acceptors. Adv. Mater. 2019, 31, 1905645. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wu, Y.; Tang, Y.; Pan, M.A.; Ma, W.; Fu, H.; Zhan, C.; Yao, J. Ternary blended fullerene-free polymer solar cells with 16.5% efficiency enabled with a higher-lumo-level acceptor to improve film morphology. Adv. Energy Mater. 2019, 9, 1901728. [Google Scholar] [CrossRef]
- Yu, R.; Yao, H.; Cui, Y.; Hong, L.; He, C.; Hou, J. Improved charge transport and reduced nonradiative energy loss enable over 16% efficiency in ternary polymer solar cells. Adv. Mater. 2019, 31, 1902302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhu, L.; Zhou, G.; Hao, T.; Qiu, C.; Zhao, Z.; Hu, Q.; Larson, B.W.; Zhu, H.; Ma, Z.; et al. Single-layered organic photovoltaics with double cascading charge transport pathways: 18% efficiencies. Nat. Commun. 2021, 12, 309. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, J.; Zhang, Z.G.; Bai, H.; Li, Y.; Zhu, D.; Zhan, X. An electron acceptor challenging fullerenes for efficient polymer solar cells. Adv. Mater. 2015, 27, 1170–1174. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, Y.; Yao, H.; Bi, P.; Hong, L.; Zhang, J.; Zu, Y.; Zhang, T.; Qin, J.; Ren, J.; et al. Single-junction organic photovoltaic cell with 19% efficiency. Adv. Mater. 2021, 33, 2102420. [Google Scholar] [CrossRef]
- Zheng, B.; Huo, L.; Li, Y. Benzodithiophenedione-based polymers: Recent advances in organic photovoltaics. NPG Asia Mater. 2020, 12, 3. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, R.; Wang, X.; Liu, T.; Li, Y.; Fu, S.; Yang, K.; Wang, Y.; Yu, C.; Jiao, L.; et al. Influence of fluorine substitution on the photovoltaic performance of wide band gap polymer donors for polymer solar cells. ACS Appl. Mater. Interfaces 2022, 14, 5740–5749. [Google Scholar] [CrossRef]
- Park, S.H.; Ahn, J.S.; Kwon, N.Y.; Diem, C.H.; Harit, A.K.; Woo, H.Y.; Cho, M.J.; Choi, D.H. Effect of fused thiophene bridges on the efficiency of non-fullerene polymer solar cells made with conjugated donor copolymers containing alkyl thiophene-3-carboxylate. Macromol. Res. 2021, 29, 435–442. [Google Scholar] [CrossRef]
- Cui, C.; Li, Y. High-performance conjugated polymer donor materials for polymer solar cells with narrow-bandgap nonfullerene acceptors. Energy Environ. Sci. 2019, 12, 3225–3246. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, C.; Lau, T.; Zhang, G.; Lu, X.; Yip, H.; So, S.K.; Beaupré, S.; et al. Fused benzothiadiazole: A building block for n-type organic acceptor to achieve high-performance organic solar cells organic solar cells. Adv. Mater. 2019, 31, 1807577. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.M.; Zhang, J.; Qin, S.; Lai, W.; Qiu, B.; Yuan, J.; Wan, Y.; Huang, W.; Li, Y. A Quinoxaline—Based D A copolymer donor achieving 17 62 efficiency of organic solar. Adv. Mater. 2021, 33, 2100474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Guo, X.; Ma, W.; Ade, H.; Hou, J. A large-bandgap conjugated polymer for versatile photovoltaic applications with high performance. Adv. Mater. 2015, 27, 4655–4660. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Zhang, Y.; Yang, B.; Hou, J. Molecular optimization enables over 13% efficiency in organic solar cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. [Google Scholar] [CrossRef]
- Handoko, S.L.; Jin, H.C.; Whang, D.R.; Kim, J.H.; Chang, D.W. Effect of cyano substituent on photovoltaic properties of quinoxaline-based polymers. J. Ind. Eng. Chem. 2020, 86, 244–250. [Google Scholar] [CrossRef]
- Handoko, S.L.; Jin, H.C.; Whang, D.R.; Putri, S.K.; Kim, J.H.; Chang, D.W. Synthesis of quinoxaline-based polymers with multiple electron-withdrawing groups for polymer solar cells. J. Ind. Eng. Chem. 2019, 73, 192–197. [Google Scholar] [CrossRef]
- Lee, S.; Ha, J.W.; Park, H.J.; Hwang, D.H. Synthesis and characterization of benzotriazole-based polymer donors with good planarity for organic photovoltaics. Macromol. Res. 2020, 903–909. [Google Scholar] [CrossRef]
- Tang, A.; Zhang, Q.; Du, M.; Li, G.; Geng, Y.; Zhang, J.; Wei, Z.; Sun, X.; Zhou, E. Molecular engineering of D−π–A copolymers based on 4,8-bis(4-chlorothiophen-2-yl)benzo [1,2-b:4,5-b′]dithiophene (BDT-T-Cl) for high-performance fullerene-free organic solar cells. Macromolecules 2019, 52, 6227–6233. [Google Scholar] [CrossRef]
- Zhou, J.; Cong, P.; Chen, L.; Zhang, B.; Geng, Y.; Tang, A.; Zhou, E. Gradually modulating the three parts of D-π-A type polymers for high-performance organic solar cells. J. Energy Chem. 2021, 62, 532–537. [Google Scholar] [CrossRef]
- Handoko, S.L.; Jeong, M.; Whang, D.R.; Kim, J.H.; Chang, D.W. High performance cyano-substituted quinoxaline-based polymers for both fullerene and nonfullerene polymer solar cells. J. Mater. Chem. A 2020, 8, 19513–19521. [Google Scholar] [CrossRef]
- Wardani, R.P.; Jeong, M.; Lee, S.W.; Whang, D.R.; Kim, J.H.; Chang, D.W. Simple methoxy-substituted quinoxaline-based D-A type polymers for nonfullerene polymer solar cells. Dye. Pigment. 2021, 192, 109346. [Google Scholar] [CrossRef]
- Intemann, J.J.; Yao, K.; Li, Y.X.; Yip, H.L.; Xu, Y.X.; Liang, P.W.; Chueh, C.C.; Ding, F.Z.; Yang, X.; Li, X.; et al. Highly efficient inverted organic solar cells through material and interfacial engineering of indacenodithieno[3,2-b]thiophene-based polymers and devices. Adv. Funct. Mater. 2014, 24, 1465–1473. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X. Optimizing electron-rich arylamine derivatives in thiophene-fused derivatives as π bridge-based hole transporting materials for perovskite solar cells. RSC Adv. 2019, 9, 24733–24741. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.; Schlegel, H.B.; Scuseria, G.; Robb, M.; Scalmani, C.J.; Barone, G.V.; Mennucci; Petersson, B.G. Gaussian 09, Revision A. 1; Gaussian Inc.: Wallingford, CT, UK, 2009; p. 34. [Google Scholar]
- Li, S.; Yan, J.; Li, C.Z.; Liu, F.; Shi, M.; Chen, H.; Russell, T.P. A non-fullerene electron acceptor modified by thiophene-2-carbonitrile for solution-processed organic solar cells. J. Mater. Chem. A 2016, 4, 3777. [Google Scholar] [CrossRef]
- Bagui, A.; Iyer, S.S.K. Increase in hole mobility in poly (3-hexylthiophene-2,5-diyl) films annealed under electric field during the solvent drying step. Org. Electron. 2014, 15, 1387–1395. [Google Scholar] [CrossRef]
- Jia, X.; Jiang, Z.; Chen, X.; Zhou, J.; Pan, L.; Zhu, F.; Sun, Z.; Huang, S. Highly efficient and air stable inverted polymer solar cells using lif-modified ITO cathode and MoO3/AgAl alloy anode. ACS Appl. Mater. Interfaces 2016, 8, 3792–3799. [Google Scholar] [CrossRef]
- Sun, C.; Pan, F.; Chen, S.; Wang, R.; Sun, R.; Shang, Z.; Qiu, B.; Min, J.; Lv, M.; Meng, L.; et al. Achieving fast charge separation and low nonradiative recombination loss by rational fluorination for high-efficiency polymer solar cells. Adv. Mater. 2019, 31, e1905480. [Google Scholar] [CrossRef] [PubMed]








(eV) a | HOMO (eV) b | LUMO (eV) b | |||
|---|---|---|---|---|---|
| IND-T-BDTF | 466, 658 | 1.65 | −5.35 | −3.59 | 1.76 |
| IND-HT-BDTF | 469, 636 | 1.65 | −5.36 | −3.58 | 1.78 |
| IND-OTT-BDTF | 471, 668 | 1.65 | −5.19 | −3.61 | 1.58 |
| Thickness (nm) | (mA/cm2) | (V) | FF (%) | PCE (%) | (Ω cm2) | (kΩ cm2) | Calculated (mA/cm2) | |
|---|---|---|---|---|---|---|---|---|
| IND-T-BDTF | 130 | 11.9 (11.8) | 0.80 (0.80) | 33.5 (33.4) | 3.18 ((3.14) | 9.64 | 0.15 | 11.6 |
| IND-HT-BDTF | 120 | 21.9 (21.8) | 0.87 (0.87) | 37.9 (37.7) | 7.23 (7.11) | 7.82 | 0.37 | 20.4 |
| IND-OTT-BDTF | 120 | 26.4 (25.2) | 0.79 (0.79) | 56.2 (56.4) | 11.7 (11.2) | 2.21 | 0.38 | 25.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Son, D.H.; Nasrun, R.F.B.; Salma, S.A.; Suh, H.; Kim, J.H. Medium Bandgap Polymers for Efficient Non-Fullerene Polymer Solar Cells—An In-Depth Study of Structural Diversity of Polymer Structure. Int. J. Mol. Sci. 2023, 24, 522. https://doi.org/10.3390/ijms24010522
Zhang S, Son DH, Nasrun RFB, Salma SA, Suh H, Kim JH. Medium Bandgap Polymers for Efficient Non-Fullerene Polymer Solar Cells—An In-Depth Study of Structural Diversity of Polymer Structure. International Journal of Molecular Sciences. 2023; 24(1):522. https://doi.org/10.3390/ijms24010522
Chicago/Turabian StyleZhang, Shimiao, Dong Hwan Son, Rahmatia Fitri Binti Nasrun, Sabrina Aufar Salma, Hongsuk Suh, and Joo Hyun Kim. 2023. "Medium Bandgap Polymers for Efficient Non-Fullerene Polymer Solar Cells—An In-Depth Study of Structural Diversity of Polymer Structure" International Journal of Molecular Sciences 24, no. 1: 522. https://doi.org/10.3390/ijms24010522