Imbalance in Bone Morphogenic Proteins 2 and 7 Is Associated with Renal and Cardiovascular Damage in Chronic Kidney Disease
Abstract
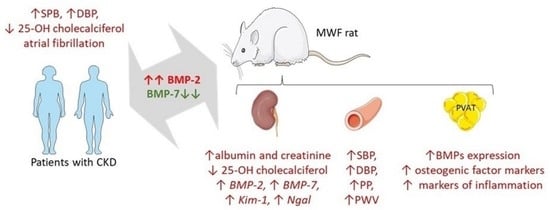
:1. Introduction
2. Results
2.1. Demographic, Clinical, and Biochemical Parameters in Study Subjects
2.2. CKD Patients Show an Imbalance in Plasma BMP Levels Associated with 25-OH-Cholecalciferol Deficiency
2.3. MWF Rats Show an Imbalance in Plasma BMP Levels Associates with 25-OH-Cholecalciferol Deficiency
2.4. MWF Rats Show an Imbalance in Renal BMP Expression Associated to Kidney Damage
2.5. MWF Rats Show an Increase in Blood Pressure and Pulse Wave Velocity Associated with the BMP-2/BMP-7 Imbalance
2.6. Perivascular Adipose Tissue from MWF Rats Shows an Altered Expression of BMP-2, BMP-7, and Profibrotic and Calcification Markers
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Animals
4.3. Plasma Determinations in Patients and Rats
4.4. RNA Extraction and Real-Time PCR (RT-qPCR)
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Mortality and Global Health Estimates: Causes of Death; Projections for 2015–2030; Projection of Death Rates. Available online: http://apps.who.int/gho/data/node.main.PROJRATEWORLD?lang=en (accessed on 13 September 2022).
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.; James, M.; Wiebe, N.; Hemmelgarn, B.; Manns, B.; Klarenbach, S.; Tonelli, M. Cause of Death in Patients with Reduced Kidney Function. J. Am. Soc. Nephrol. 2015, 26, 2504–2511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Blacher, J.; Guerin, A.P.; Pannier, B.; Marchais, S.J.; Safar, M.E.; London, G.M. Impact of aortic stiffness on survival in end-stage renal disease. Circulation 1999, 99, 2434–2439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanoli, L.; Lentini, P.; Briet, M.; Castellino, P.; House, A.A.; London, G.M.; Malatino, L.; McCullough, P.A.; Mikhailidis, D.P.; Boutouyrie, P. Arterial Stiffness in the Heart Disease of CKD. J. Am. Soc. Nephrol. 2019, 30, 918–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giachelli, C.M. The emerging role of phosphate in vascular calcification. Kidney Int. 2009, 75, 890–897. [Google Scholar] [CrossRef] [Green Version]
- Guérin, A.P.; London, G.M.; Marchais, S.J.; Metivier, F. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol. Dial. Transplant. 2000, 15, 1014–1021. [Google Scholar] [CrossRef] [Green Version]
- Davies, M.R.; Hruska, K.A. Pathophysiological mechanisms of vascular calcification in end-stage renal disease. Kidney Int. 2001, 60, 472–479. [Google Scholar] [CrossRef] [Green Version]
- Hinck, A.P. Structural studies of the TGF-βs and their receptors-insights into evolution of the TGF-β superfamily. FEBS Lett. 2012, 586, 1860–1870. [Google Scholar] [CrossRef] [Green Version]
- Ozkaynak, E.; Rueger, D.C.; Drier, E.A.; Corbett, C.; Ridge, R.J.; Sampath, T.K.; Oppermann, H. OP-1 cDNA encodes an osteogenic protein in the TGF-beta family. EMBO J. 1990, 9, 2085–2093. [Google Scholar] [CrossRef]
- Dudley, A.T.; Lyons, K.M.; Robertson, E.J. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995, 9, 2795–2807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, G.; Hofmann, C.; Bronckers, A.L.; Sohocki, M.; Bradley, A.; Karsenty, G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995, 9, 2808–2820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bramlage, C.P.; Tampe, B.; Koziolek, M.; Maatouk, I.; Bevanda, J.; Bramlage, P.; Ahrens, K.; Lange, K.; Schmid, H.; Cohen, C.D.; et al. Bone Morphogenetic Protein (BMP)-7 expression is decreased in human hypertensive nephrosclerosis. BMC Nephrol. 2010, 11, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duranay, M.; Segall, L.; Sen, N.; Yilmaz, F.M.; Cetin, M.; Isleyen, A.; Kanbay, M.; Covic, A. Bone morphogenic protein-7 serum level decreases significantly in patients with contrast-induced nephropathy. Int. Urol. Nephrol. 2011, 43, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Manson, S.R.; Song, J.B.; Guo, Q.; Liapis, H.; Austin, P.F. Cell type specific changes in BMP-7 expression contribute to the progression of kidney disease in patients with obstructive uropathy. J. Urol. 2015, 193, 1860–1869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spanjol, J.; Djordjevic, G.; Markić, D.; Fuckar, D.; Krpina, K.; Bobinac, D. Bone morphogenetic protein-7 expression in human pyelonephritis. Coll. Antropol. 2010, 34, 61–64. [Google Scholar]
- Turk, T.; Leeuwis, J.W.; Gray, J.; Torti, S.V.; Lyons, K.M.; Nguyen, T.Q.; Goldschmeding, R. BMP signaling and podocyte markers are decreased in human diabetic nephropathy in association with CTGF overexpression. J. Histochem. Cytochem. 2009, 57, 623–631. [Google Scholar] [CrossRef] [Green Version]
- Vukicevic, S.; Basic, V.; Rogic, D.; Basic, N.; Shih, M.S.; Shepard, A.; Jin, D.; Dattatreyamurty, B.; Jones, W.; Dorai, H.; et al. Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J. Clin. Investig. 1998, 102, 202–214. [Google Scholar] [CrossRef] [Green Version]
- Hruska, K.A.; Guo, G.; Wozniak, M.; Martin, D.; Miller, S.; Liapis, H.; Loveday, K.; Klahr, S.; Sampath, T.K.; Morrissey, J. Osteogenic protein-1 prevents renal fibrogenesis associated with ureteral obstruction. Am. J. Physiol. Physiol. 2000, 279, F130–F143. [Google Scholar] [CrossRef]
- Sugimoto, H.; Grahovac, G.; Zeisberg, M.; Kalluri, R. Renal fibrosis and glomerulosclerosis in a new mouse model of diabetic nephropathy and its regression by bone morphogenic Protein-7 and advanced glycation end product inhibitors. Diabetes 2007, 56, 1825–1833. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Hirschberg, R. BMP7 antagonizes TGF-beta -dependent fibrogenesis in mesangial cells. Am. J. Physiol. Renal. Physiol. 2003, 284, F1006–F1013. [Google Scholar] [CrossRef] [PubMed]
- Dalfino, G.; Simone, S.; Porreca, S.; Cosola, C.; Balestra, C.; Manno, C.; Schena, F.P.; Grandaliano, G.; Pertosa, G. Bone morphogenetic protein-2 may represent the molecular link between oxidative stress and vascular stiffness in chronic kidney disease. Atherosclerosis 2010, 211, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Boström, K.; Watson, K.E.; Horn, S.; Wortham, C.; Herman, I.M.; Demer, L. Bone morphogenetic protein expression in human atherosclerotic lesions. J. Clin. Investig. 1993, 91, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Duan, D.; O’Neill, K.; Wolisi, G.; Koczman, J.; LaClair, R.; Moe, S. The mechanisms of uremic serum-induced expression of bone matrix proteins in bovine vascular smooth muscle cells. Kidney Int. 2006, 70, 1046–1053. [Google Scholar] [CrossRef] [Green Version]
- Rong, S.; Zhao, X.; Jin, X.; Zhang, Z.; Chen, L.; Zhu, Y.; Yuan, W. Vascular calcification in chronic kidney disease is induced by bone morphogenetic protein-2 via a mechanism involving the Wnt/β-catenin pathway. Cell Physiol. Biochem. 2014, 34, 2049–2060. [Google Scholar] [CrossRef]
- Pulido-Olmo, H.; García-Prieto, C.F.; Álvarez-Llamas, G.; Barderas, M.G.; Vivanco, F.; Aranguez, I.; Somoza, B.; Segura, J.; Kreutz, R.; Fernández-Alfonso, M.S.; et al. Role of matrix metalloproteinase-9 in chronic kidney disease: A new biomarker of resistant albuminuria. Clin. Sci. 2016, 130, 525–538. [Google Scholar] [CrossRef]
- Ruiz-Hurtado, G.; Condezo-Hoyos, L.; Pulido-Olmo, H.; Aranguez, I.; Gónzalez, M.D.C.; Arribas, S.; Cerezo, C.; Segura, J.; Praga, M.; Fernández-Alfonso, M.S.; et al. Development of albuminuria and enhancement of oxidative stress during chronic renin–angiotensin system suppression. J. Hypertens. 2014, 32, 2082–2091. [Google Scholar] [CrossRef]
- Gil-Ortega, M.; García-Prieto, C.F.; Ruiz-Hurtado, G.; Steireif, C.; González, M.C.; Schulz, A.; Kreutz, R.; Fernández-Alfonso, M.S.; Arribas, S.; Somoza, B. Genetic predisposition to albuminuria is associated with increased arterial stiffness: Role of elastin. J. Cereb. Blood Flow Metab. 2015, 172, 4406–4418. [Google Scholar] [CrossRef] [Green Version]
- Almanzar, M.M.; Frazier, K.S.; Dube, P.H.; Piqueras, A.I.; Jones, W.K.; Charette, M.F.; Paredes, A.L. Osteogenic protein-1 mRNA expression is selectively modulated after acute ischemic renal injury. J. Am. Soc. Nephrol. 1998, 9, 1456–1463. [Google Scholar] [CrossRef]
- Simon, M.; Feliers, D.; Arar, M.; Bhandari, B.; Abboud, H.E. Cloning of the 5′-flanking region of the murine bone morphogenetic protein-7 gene. Mol. Cell Biochem. 2002, 233, 31–37. [Google Scholar] [CrossRef]
- Tuğlular, S.; Yavuz, D.G.; Çakalağaoğlu, F.; Çıtak, L.; Arıkan, H.; Koçak, H.; Özener, Ç.; Akoğlu, E. Cyclosporine-a induced nephrotoxicity is associated with decreased renal bone morphogenetic protein-7 expression in rats. Transplant. Proc. 2004, 36, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.G.; Perkovic, V.; Woodward, M.; Chalmers, J.; Li, Q.; Hillis, G.S.; Azari, D.Y.; Jun, M.; Poulter, N.; Hamet, P.; et al. Circulating bone morphogenetic protein-7 and transforming growth factor-β1 are better predictors of renal end points in patients with type 2 diabetes mellitus. Kidney Int. 2013, 83, 278–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, S.; Davies, M.; Lund, R.; Saab, G.; Hruska, K.A. Function and effect of bone morphogenetic protein-7 in kidney bone and the bone-vascular links in chronic kidney disease. Eur. J. Clin. Investig. 2006, 36, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Standke, D.; Kovacevic, L.; Mostler, M.; Kossmehl, P.; Stoll, M.; Kreutz, R. A major gene locus links early onset albuminuria with renal interstitial fibrosis in the MWF rat with polygenetic albuminuria. J. Am. Soc. Nephrol. 2003, 14, 3081–3089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stompór, T. Coronary artery calcification in chronic kidney disease: An update. World J. Cardiol. 2014, 6, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Palmiero, G.; De Blasio, A.P.; Balletta, M.M.; Andreucci, V.E. Coronary artery calcification in patients with CRF not undergoing dialysis. Am. J. Kidney Dis. 2004, 44, 1024–1030. [Google Scholar] [CrossRef]
- Sigrist, M.K.; Taal, M.W.; Bungay, P.; McIntyre, C.W. Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2007, 2, 1241–1248. [Google Scholar] [CrossRef] [Green Version]
- Barreto, D.V.; Barreto, F.C.; Liabeuf, S.; Temmar, M.; Boitte, F.; Choukroun, G.; Fournier, A.; Massy, Z.A. Vitamin D affects survival independently of vascular calcification in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 1128–1135. [Google Scholar] [CrossRef] [Green Version]
- Verheule, S.; Sato, T.; Everett, T., IV; Engle, S.K.; Otten, D.; Rubart-von der Lohe, M.; Nakajima, H.O.; Nakajima, H.; Field, L.J.; Olgin, J.E. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ. Res. 2004, 94, 1458–1465. [Google Scholar] [CrossRef] [Green Version]
- Himmelfarb, J.; Stenvinkel, P.; Ikizler, T.A.; Hakim, R.M. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002, 62, 1524–1538. [Google Scholar] [CrossRef] [Green Version]
- Steireif, C.; García-Prieto, C.F.; Ruiz-Hurtado, G.; Pulido-Olmo, H.; Aranguez, I.; Gil-Ortega, M.; Somoza, B.; Schönfelder, G.; Schulz, A.; Fernández-Alfonso, M.S.; et al. Dissecting the genetic predisposition to albuminuria and endothelial dysfunction in a genetic rat model. J. Hypertens. 2013, 31, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, M.K.; Buikema, J.H.; van Veldhuisen, D.J.; Koster, J.; van der Velden, J.; Hamdani, N.; Hillege, J.L.; Schoemaker, R.G. Increased cardiovascular risk in rats with primary renal dysfunction; mediating role for vascular endothelial function. Basic Res. Cardiol. 2012, 107, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boon, M.R.; van der Horst, G.; van der Pluijm, G.; Tamsma, J.T.; Smit, J.W.; Rensen, P.C. Bone morphogenetic protein 7: A broad-spectrum growth factor with multiple target therapeutic potency. Cytokine Growth Factor Rev. 2011, 22, 221–229. [Google Scholar] [CrossRef]
- Zeisberg, M. Bone morphogenic protein-7 and the kidney: Current concepts and open questions. Nephrol. Dial. Transplant. 2006, 21, 568–573. [Google Scholar] [CrossRef] [Green Version]
- Gould, S.E.; Day, M.; Jones, S.S.; Dorai, H. BMP-7 regulates chemokine, cytokine, and hemodynamic gene expression in proximal tubule cells. Kidney Int. 2002, 61, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Zeisberg, M.; Hanai, J.; Sugimoto, H.; Mammoto, T.; Charytan, D.; Strutz, F.; Kalluri, R. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 2003, 9, 964–968. [Google Scholar] [CrossRef]
- Zeisberg, M.; Bottiglio, C.; Kumar, N.; Maeshima, Y.; Strutz, F.; Müller, G.A.; Kalluri, R. Bone morphogenic protein-7 inhibits progression of chronic renal fibrosis associated with two genetic mouse models. Am. J. Physiol. Physiol. 2003, 285, F1060–F1067. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-P.; Dong, J.-Z.; Xiong, L.-J.; Shi, K.-Q.; Zou, Z.-L.; Zhang, S.-N.; Cao, S.-T.; Lin, Z.; Chen, Y.-P. BMP-7 attenuates liver fibrosis via regulation of epidermal growth factor receptor. Int. J. Clin. Exp. Pathol. 2014, 7, 3537–3547. [Google Scholar]
- Chen, Y.P.; Yang, T.; Chen, S.L.; Lu, X.J.; Shen, C.Y.; Liu, Y. Bone morphogenetic protein 7 suppresses the progression of hepatic fibrosis and regulates the expression of gremlin and transforming growth factor β1. Mol. Med. Rep. 2012, 6, 246–252. [Google Scholar] [CrossRef]
- Merino, D.; Villar, A.V.; García, R.; Tramullas, M.; Ruiz, L.; Ribas, C.; Cabezudo, S.; Nistal, J.F.; Hurlé, M.A. BMP-7 attenuates left ventricular remodelling under pressure overload and facilitates reverse remodelling and functional recovery. Cardiovasc. Res. 2016, 110, 331–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.-L.; Shao, J.-S.; Charlton-Kachigian, N.; Loewy, A.P.; Towler, D.A. Msx2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J. Biol. Chem. 2003, 278, 45969–45977. [Google Scholar] [CrossRef] [Green Version]
- Jang, W.-G.; Kim, E.-J.; Kim, D.-K.; Ryoo, H.-M.; Lee, K.-B.; Kim, S.-H.; Choi, H.-S.; Koh, J.-T. BMP2 protein regulates osteocalcin expression via Runx2-mediated Atf6 gene transcription. J. Biol. Chem. 2012, 287, 905–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.H.; Kim, Y.J.; Kim, H.J.; Park, H.D.; Kang, A.R.; Kyung, H.M.; Sung, J.H.; Wozney, J.M.; Ryoo, H.M. BMP-2-induced Runx2 expression is mediated by Dlx5, and TGF-beta 1 opposes the BMP-2-induced osteoblast differentiation by suppression of Dlx5 expression. J. Biol. Chem. 2003, 278, 34387–34394. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Alfonso, M.S.; Somoza, B.; Tsvetkov, D.; Kuczmanski, A.; Dashwood, M.; Gil-Ortega, M. Role of Perivascular Adipose Tissue in Health and Disease. Compr. Physiol. 2017, 8, 23–59. [Google Scholar]
- Gil-Ortega, M.; Somoza, B.; Huang, Y.; Gollasch, M.; Fernández-Alfonso, M.S. Regional differences in perivascular adipose tissue impacting vascular homeostasis. Trends Endocrinol. Metab. 2015, 26, 367–375. [Google Scholar] [CrossRef]
- Chattopadhyay, T.; Singh, R.R.; Gupta, S.; Surolia, A. Bone morphogenetic protein-7 (BMP-7) augments insulin sensitivity in mice with type II diabetes mellitus by potentiating PI3K/AKT pathway. BioFactors 2017, 43, 195–209. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612, Correction in Ann. Intern. Med. 2011, 155, 408. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104, Correction in Eur. Heart. J. 2019, 40, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930, Erratum in J. Clin. Endocrinol. Metab. 2011, 96, 3908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosson, E.; Herisse, M.; Laude, D.; Thomas, F.; Valensi, P.; Attali, J.-R.; Safar, M.E.; Dabiré, H. Aortic stiffness and pulse pressure amplification in Wistar-Kyoto and spontaneously hypertensive rats. Am. J. Physiol. Circ. Physiol. 2007, 292, H2506–H2512. [Google Scholar] [CrossRef] [PubMed]






| Control (n = 26) | Stage I (n = 32) | Stage II (n = 37) | Stage III (n = 26) | |
|---|---|---|---|---|
| Number of female (%) | 18 (69.2%) | 13 (40.6%) | 15 (40.5%) | 10 (38.5%) |
| Age (18–65 years) (%) | 26 (100.0%) | 23 (71.9%) | 16 (43.2%) | 2 (7.7%) |
| Age (years) | 46.4 ± 7.3 | 57.8 ± 8.2 | 64.9 ± 8.8 | 72.0 ± 10.1 |
| Kidney function markers | ||||
| Creatinine (mg/dL) | 0.77 ± 0.2 | 0.78 ± 0.1 | 0.97 ± 0.1 * | 1.57 ± 0.6 *# |
| eGFR (ml/min/1.73 m2) | 113.7 ± 6.1 | 95.25 ± 5.2 | 74.08 ± 9.0 * | 44.50 ± 13.4 * |
| Proteinuria (>30 mg/dL). (N/%) | -- | 9 (28.1%) | 25 (67.7%) | 6 (23.1%) |
| Albumin (mg/dL) | -- | 4.56 ± 0.8 | 4.55 ± 0.7 | 4.36 ± 0.6 |
| Albumin/creatinine | -- | 26.34 ± 35.8 | 39.07 ± 58.9 | 45.4 ± 70.1 |
| NGAL (ng/mL) | -- | 43.09 ± 27.35 | 60.08 ± 39.01 | 142.87 ± 160.1 ***## |
| Uric acid (mg/dL) | 5.36 ± 1.1 | 5.55 ± 1.4 | 6.11 ± 1.8 | 6.92 ± 2.0 |
| Anthropometric measurements | ||||
| BMI (18.5–24.9 kg/m2) | 24 (92.3%) | 4 (12.5%) | 8 (21.6%) | 11 (42.3%) |
| BMI (25–29.9 kg/m2) | 2 (7.7%) | 13 (40.6%) | 12 (32.4%) | 8 (30.8%) |
| BMI (>30 kg/m2) | 0 (0.0%) | 15 (46.9%) | 17 (45.9%) | 7 (26.9%) |
| BMI (kg/m2) | 24.21 ± 6.8 | 30.15 ± 4.5 | 30.38 ± 5.0 | 30.27 ± 5.9 |
| Metabolic indicators | ||||
| Blood glucose (mg/dL) | 98.23 ± 4.3 | 111.37 ± 19.0 | 115.81 ± 25.9 | 113.19 ± 21.5 |
| Hb1AC (%) | 5.1 ± 0.2 | 5.80 ± 0.6 | 6.10 ± 0.7 | 5.81 ± 0.7 |
| Cholesterol (mg/dL) | 191.33 ± 21.7 | 184.75 ± 35.7 | 171.08 ± 33.5 | 155.20 ± 39.0 |
| Triglycerides (mg/dL) | 117.93 ± 18.7 | 114.56 ± 39.1 | 115.97 ± 37.7 | 130.00 ± 62.5 |
| HDL (mg/dL) | 60.61 ± 15.6 | 55.63 ± 15.6 | 51.46 ± 12.2 | 48.01 ± 14.7 |
| LDL (mg/dL) | 143.24 ± 14.2 | 106.18 ± 33.2 | 96.32 ± 26.9 | 78.50 ± 31.9 |
| Cardiovascular parameters | ||||
| Arterial hypertension. n (%) | 0 (0.0%) | 32 (100.0%) | 37 (100.0%) | 25 (96.2%) |
| SBP (mmHg) | 127.33 ± 11.3 | 137.70 ± 14.8 | 140.29 ± 19.0 | 139.23 ± 22.4 |
| DBP (mmHg) | 74.09 ± 10.6 | 83.09 ± 9.2 | 81.15 ± 9.1 | 82.30 ± 9.0 |
| Gene | Accession Number | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|---|
| Rn Kim-1 | NM_173149.2 | ATTGTTGCCGAGTGGAGAT | TGTGGTTGTGGGTCTTGTAGT |
| Rn Ngal | NM_130741.1 | GGCCGACACTGACTACGACC | GCCCCTTGGTTCTTCCGTAC |
| Rn BMP-2 | NM_017178.2 | CCCCTATATGCTCGACCTGTACC | TGAAAGTTCCTCGATGGCTTCT |
| Rn BMP-7 | NM_001191856.2 | GAGGGCTGGTTGGTATTTGACA | AACTTGGGGTTGATGCTCTGC |
| Rn Runx2 | NM_001278484.2 | CACCGTGTCAGCAAAACTTCTTT | CTACGTCGCTCATCTTGC |
| Rn ALP | NM_013059.2 | ATGCACAACATCAAGGACATCG | CATCAGTTCTGTTCTTGGGGTACAT |
| Rn Bglap | NM_013414.1 | GCTACCTCAACAATGGACTTGGA | GAGCTCACACACCTCCCTGTG |
| Rn Col1A1 | NM_053304.1 | GGATGCCATCAAGGTCTACTGC | TGAGTGGGGAACACACAGGTCT |
| Rn GAPH | NM_017008.4 | AAGGCTGAGAAATGGGAAGCTC | CCATTTGATGTTAGCGGGATCT |
| Rn ATPAF-1 | NM_001107959.1 | GATCTCTCCAAGAAGCTGCAAG | AAGATGACCCCAAGGCATTTTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzano-Lista, F.J.; Sanz-Gómez, M.; González-Moreno, D.; Vega-Martín, E.; Gil-Ortega, M.; Schulz, A.; Rubio, M.Á.; Ruiz-Hurtado, G.; Ruilope, L.M.; Aránguez, I.; et al. Imbalance in Bone Morphogenic Proteins 2 and 7 Is Associated with Renal and Cardiovascular Damage in Chronic Kidney Disease. Int. J. Mol. Sci. 2023, 24, 40. https://doi.org/10.3390/ijms24010040
Manzano-Lista FJ, Sanz-Gómez M, González-Moreno D, Vega-Martín E, Gil-Ortega M, Schulz A, Rubio MÁ, Ruiz-Hurtado G, Ruilope LM, Aránguez I, et al. Imbalance in Bone Morphogenic Proteins 2 and 7 Is Associated with Renal and Cardiovascular Damage in Chronic Kidney Disease. International Journal of Molecular Sciences. 2023; 24(1):40. https://doi.org/10.3390/ijms24010040
Chicago/Turabian StyleManzano-Lista, Francisco Javier, Marta Sanz-Gómez, Daniel González-Moreno, Elena Vega-Martín, Marta Gil-Ortega, Angela Schulz, Miguel Ángel Rubio, Gema Ruiz-Hurtado, Luis Miguel Ruilope, Isabel Aránguez, and et al. 2023. "Imbalance in Bone Morphogenic Proteins 2 and 7 Is Associated with Renal and Cardiovascular Damage in Chronic Kidney Disease" International Journal of Molecular Sciences 24, no. 1: 40. https://doi.org/10.3390/ijms24010040