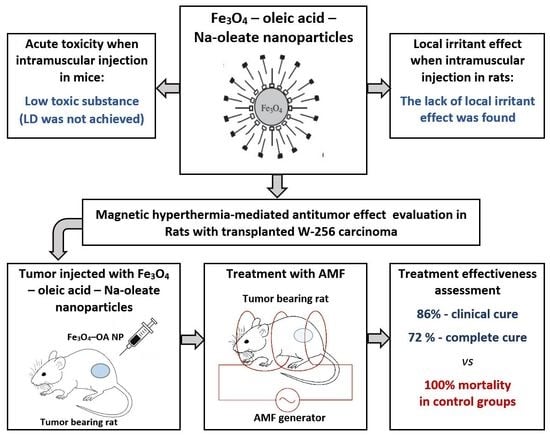
Magnetic Hyperthermia Nanoarchitectonics via Iron Oxide Nanoparticles Stabilised by Oleic Acid: Anti-Tumour Efficiency and Safety Evaluation in Animals with Transplanted Carcinoma
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteristics of Magnetic Nanoparticles
2.2. Acute Toxicity
2.3. Local Irritant Effect and Effect on Blood
2.3.1. Local Irritant Effect
2.3.2. Effect on Blood
2.4. Antineoplastic Effect of MHT
2.4.1. Study of Heating and Growth of W256 Adenocarcinoma
2.4.2. Tumour Growth
2.4.3. Blood Analysis
2.4.4. Histological Observation of Region of Treatment
2.4.5. Influence of HT on Survival Period of Rats
3. Materials and Methods
3.1. Reagents
3.2. Synthesis and Characterisation of Magnetic Nanoparticles
3.3. Animals
3.4. Acute (Single-Dose) Toxicity of Fe3O4-OA-NP
3.4.1. Intramuscular Administration
3.4.2. Intraperitoneal Administration
3.5. Study of Local Irritant Action
3.6. Antineoplastic Effect and Safety of MHT
3.6.1. Establishment of Animal Tumour Model
3.6.2. Experimental Groups
3.6.3. Induction of HT
3.6.4. Assessment of Tumour Growth and Rat Survival Time
3.7. Euthanasia
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adam, C.; Janusz, S.; Kubaszewska, M.; Marek, K. Hyperthermia—Description of a method and a review of clinical applications. Rep. Pract. Oncol. Radiother. 2007, 12, 267–275. [Google Scholar]
- Zhang, X.-D.; Wu, D.; Shen, X.; Chen, J.; Sun, Y.-M.; Liu, X.-P.; Liang, X.-J. Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials 2012, 33, 6408–6419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Liang, P.; Yu, X.; Cheng, Z.; Han, Z.; Mu, M.; Wang, X. US-guided Percutaneous Microwave Ablation of Renal Cell Carcinoma: Intermediate-term Results. Radiology 2012, 263, 900–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.; Cho, J.; Kim, N.; Kim, D.; Lee, E.; Cheon, C.; Kwon, Y. High-frequency microwave ablation method for enhanced cancer treatment with minimized collateral damage. Int. J. Cancer 2011, 129, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- Perigo, E.A.; Hemery, G.; Sandre, O.; Ortega, D.; Garaio, E.; Plazaola, F.; Teran, F.J. Fundamentals and advances in magnetic hyperthermia. Appl. Phys. Rev. 2015, 2, 041302. [Google Scholar] [CrossRef] [Green Version]
- Vilas-Boas, V.; Espina, B.; Kolen’ko, Y.V.; Banobre-Lopez, M.; Brito, M.; Martins, V.; Duarte, J.A.; Petrovykh, D.Y.; Freitas, P.; Carvalho, F. Effectiveness and Safety of a Nontargeted Boost for a CXCR4-Targeted Magnetic Hyperthermia Treatment of Cancer Cells. ACS Omega 2019, 4, 1931–1940. [Google Scholar] [CrossRef]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer. 2014, 14, 199–208. [Google Scholar] [CrossRef]
- Saleem, J.; Patrick, F.; Declan, S.; John, H. Review of current thermal ablation treatment for lung cancer and the potential of electrochemotherapy as a means for treatment of lung tumours. Cancer Treat. Rev. 2013, 39, 862–871. [Google Scholar]
- Elengoe, A.; Hamdan, S. Heat sensitivity between human normal liver (WRL-68) and breast cancer (MCF-7) cell lines. J. Biotechnol. Lett. 2013, 1, 45–50. [Google Scholar]
- Armour, E.P.; Mceachern, D.; Wang, Z.; Corry, P.M.; Martinez, A. Sensitivity of human cells to mild hyperthermia. Cancer Res. 1993, 53, 2740–2744. [Google Scholar]
- Brero, F.; Albino, M.; Antoccia, A.; Arosio, P.; Avolio, M.; Berardinelli, F.; Bettega, D.; Calzolari, P.; Ciocca, M.; Corti, M.; et al. Hadron Therapy, Magnetic Nanoparticles and Hyperthermia: A Promising Combined Tool for Pancreatic Cancer Treatment. Nanomaterials 2020, 10, 1919. [Google Scholar] [CrossRef] [PubMed]
- Labavić, D.; Ladjimi, M.T.; Courtade, E.; Pfeuty, B.; Thommen, Q. Mammalian cell sensitivity to hyperthermia in various cell lines: A new universal and predictive description. Int. J. Hyperth. 2020, 37, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.H. Tumour progression and the nature of cancer. Br. J. Cancer 1991, 64, 631–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, M.M.; Fusenig, N.E. Friends or foes—Bipolar effects of the tumour stroma in cancer. Nat. Rev. Cancer 2004, 4, 839–849. [Google Scholar] [CrossRef]
- Ryan, T.P.; Brace, C.L. Interstitial microwave treatment for cancer: Historical basis and current techniques in antenna design and performance. Int. J. Hyperth. 2017, 33, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsakok, M.T.; Little, M.W.; Hynes, G.; Millington, R.S.; Boardman, P.; Gleeson, F.V.; Anderson, E.M. Local control, safety, and survival following image guided percutaneous microwave thermal ablation in primary lung malignancy. Clin. Radiol. 2019, 74, 80.e19–80.e26. [Google Scholar] [CrossRef] [PubMed]
- Klapperich, M.E.; Abel, E.J.; Ziemlewicz, T.J.; Best, S.; Lubner, M.G.; Nakada, S.Y.; Hinshaw, J.L.; Brace, C.L.; Lee, F.T., Jr.; Wells, S.A. Technique on Efficacy and Complications after Percutaneous Microwave Ablation of Stage T1a Renal Cell Carcinoma: A Single-Center, Retrospective Study. Radiology 2017, 284, 272–280. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S.; Müller, R.; Zeisberger, M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J. Phys. Condens. Matter. 2006, 18, S2919. [Google Scholar] [CrossRef]
- Salunkhe, A.B.; Khot, V.M.; Pawar, S.H. Magnetic hyperthermia with magnetic nanoparticles: A status review. Curr. Top. Med. Chem. 2014, 14, 572–594. [Google Scholar] [CrossRef]
- Obaidat, I.M.; Issa, B.; Haik, Y. Magnetic properties of magnetic nanoparticles for efficient hyperthermia. Nanomaterials 2015, 5, 63–89. [Google Scholar] [CrossRef] [Green Version]
- Stafford, S.; Garcia, R.S.; Gun’ko, Y.K. Multimodal Magnetic-Plasmonic Nanoparticles for Biomedical Applications. Appl. Sci. 2018, 8, 97. [Google Scholar] [CrossRef] [Green Version]
- Iacovita, C.; Florea, A.; Scorus, L.; Pall, E.; Dudric, R.; Moldovan, A.I.; Stiufiuc, R.; Tetean, R.; Lucaciu, C.M. Hyperthermia, Cytotoxicity, and Cellular Uptake Properties of Manganese and Zinc Ferrite Magnetic Nanoparticles Synthesized by a Polyol-Mediated Process. Nanomaterials 2019, 9, 1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gun’ko, Y.K. Nanoparticles in Bioimaging. Nanomaterials 2016, 6, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardo, A.; Pelaz, B.; Gallo, J.; Banobre-Lopez, M.; Parak, W.J.; Barbosa, S.; del Pino, P.; Taboada, P. Synthesis, Characterization, and Evaluation of Superparamagnetic Doped Ferrites as Potential Therapeutic Nanotools. Chem. Mater. 2020, 32, 2220–2231. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lee, S.-H.; Kim, K.-N.; Kim, K.-M.; Shim, I.-B.; Lee, Y.-K. Cytotoxicity of ferrite particles by MTT and agar diffusion methods for hyperthermic application. J. Magn. Magn. Mat. 2005, 293, 287–292. [Google Scholar] [CrossRef]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic Nanoparticles: Surface Effects and Properties Related to Biomedicine Applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef] [Green Version]
- Hanini, A.; Massoudi, M.E.; Gavard, J.; Kacem, K.; Ammar, S.; Souilem, O. Nanotoxicological study of polyol-made cobalt-zinc ferrite nanoparticles in rabbit. Environ. Toxicol. Pharmacol. 2016, 45, 321. [Google Scholar] [CrossRef]
- Vidiasheva, I.V.; Abalymov, A.A.; Kurochkin, M.A.; Mayorova, O.A.; Lomova, M.V.; German, S.V.; Khalenkow, D.N.; Zharkov, M.N.; Gorin, D.A.; Skirtach, A.G.; et al. Transfer of cells with uptaken nanocomposite, magnetite-nanoparticle functionalized capsules with electromagnetic tweezers. Biomater. Sci. 2018, 6, 2219–2229. [Google Scholar] [CrossRef]
- Zharkov, M.N.; Brodovskaya, E.P.; Kulikov, O.A.; Gromova, E.V.; Ageev, V.P.; Atanova, A.V.; Kozyreva, Z.V.; Tishin, A.M.; Pyatakov, A.P.; Pyataev, N.A.; et al. Enhanced cytotoxicity caused by AC magnetic field for polymer microcapsules containing packed magnetic nanoparticles. Colloids Surf. B Biointerfaces 2021, 199, 111548. [Google Scholar] [CrossRef]
- El-Boubbou, K. Magnetic iron oxide nanoparticles as drug carriers: Clinical relevance. Nanomedicine 2018, 13, 953–971. [Google Scholar] [CrossRef]
- Mahmoudi, K.; Bouras, A.; Bozec, D.; Ivkov, R.; Hadjipanayis, C. Magnetic hyperthermia therapy for the treatment of glioblastoma: A review of the therapy’s history, efficacy and application in humans. Int. J. Hyperth. 2018, 34, 1316–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldoefner, N.; Jordan, A. Agglomerating Magnetic Alkoxysilane—Coated Nanoparticles. T.N.C. Magforce ag, the Nanotherm Therapy. U.S. Patent 9,962,442 B2, 8 May 2018. [Google Scholar]
- Grüttner, C.; Müller, K.; Teller, J.; Westphal, F. Synthesis and functionalisation of magnetic nanoparticles for hyperthermia applications. Int. J. Hyperth. 2013, 29, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Grauer, O.; Jaber, M.; Hess, K.; Weckesser, M.; Schwindt, W.; Maring, S.; Wölfer, J.; Stummer, W. Combined intracavitary thermotherapy with iron oxide nanoparticles and radiotherapy as local treatment modality in recurrent glioblastoma patients. J. Neurooncol. 2019, 141, 83–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Miao, Y.Q.; Yu, B.Z.; Ma, P.; Li, L.; Fan, H.M. Large-Scale, Facile Transfer of Oleic Acid-Stabilized Iron Oxide Nanoparticles to the Aqueous Phase for Biological Applications. Langmuir 2017, 33, 1662–1669. [Google Scholar] [CrossRef]
- Soares, P.; Laia, C.; Carvalho, A.; Pereira, L.; Coutinho, J.; Ferreira, I.; Novo, C.; Borges, J. Iron oxide nanoparticles stabilized with a bilayer of oleic acid 2 for magnetic hyperthermia and MRI applications. Appl. Surf. Sci. 2016, 383, 240–247. [Google Scholar] [CrossRef]
- Fernandez-Bertolez, N.; Costa, C.; Brandao, F.; Kilic, G.; Teixeira, J.P.; Pasaro, E.; Laffon, B.; Valdiglesias, V. Neurotoxicity assessment of oleic acid-coated iron oxide nanoparticles in SH-SY5Y cells. Toxicology 2018, 406–407, 81–91. [Google Scholar] [CrossRef]
- Zablotskaya, A.; Segal, I.; Maiorov, M.; Zablotsky, D.; Blums, E. Iron oxide/oleic acid magnetic nanoparticles possessing biologically active choline derivatives: Synthesis, morphology, antitumor, and antimicrobial properties. In Inorganic Frameworks as Smart Nanomedicines; Grumezescu, A.M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 279–316. [Google Scholar]
- Wetterskog, E.; Tai, C.W.; Grins, J.; Bergstrom, L.; Salazar Alvarez, G. Anomalous magnetic properties of nanoparticles arising from defect structures: Topotaxial oxidation of Fe(1-x)O|Fe(3-δ)O4 core|shell nanocubes to single-phase particles. ACS Nano 2013, 7, 7132–7144. [Google Scholar] [CrossRef]
- Berezovskaya, I.V. Classification of chemicals according to the parameters of acute toxicity with parenteral routes of administration. Pharm. Chem. J. 2003, 37, 139–141. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, Q.; Zhang, R.; Wang, Y.; Niu, F.; Wang, W.; Sun, D.; Wang, A. ITRAQ-Based Proteomics Analysis of Acute Lung Injury Induced by Oleic Acid in Mice. Cell. Physiol. Biochem. 2017, 44, 1949–1964. [Google Scholar] [CrossRef]
- Xing, C.H.; Zhu, M.H.; Cai, M.Z.; Liu, P.; Xu, G.D.; Wu, S.H. Developmental characteristics and response to iron toxicity of root border cells in rice seeding. J. Zhejiang Univ. Sci. B 2008, 9, 261–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.-L.; Feng, Y.; Li, X.-L.; Wei, Y.-Y.; Yang, X.-E. Availability and toxicity of Fe(II) and Fe(III) in Caco-2 cells. Zhejiang Univ. Sci. B 2008, 9, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Patsula, V.; Tulinska, J.; Trachtova, S.; Kuricova, M.; Liskova, A.; Spanova, A.; Ciampor, F.; Vavra, I.; Rittich, B.; Ursinyova, M.; et al. Toxicity evaluation of monodisperse PEGylated magnetic nanoparticles for nanomedicine. Nanotoxicology 2019, 13, 510–526. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.C.; Fleming, M.D.; Gunshin, H. Iron transport across biologic membranes. Nutr. Rev. 1999, 57, 114–123. [Google Scholar] [CrossRef]
- Yanatori, I.; Yasui, Y.; Tabuchi, M.; Kishi, F. Chaperone protein involved in transmembrane transport of iron. Biochem. J. 2014, 462, 25–37. [Google Scholar] [CrossRef]
- Richter, K.; Haslbeck, M.; Buchner, J. The heat shock response: Life on the verge of death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef]
- Mello, P.D.; Bian, S.; Savio, L.E.B.; Zhang, H.H.; Zhang, J.P.; Junger, W.; Wink, M.R.; Lenz, G.; Buffon, A.; Wu, Y.; et al. Hyperthermia and associated changes in membrane fluidity potentiate P2X7 activation to promote tumor cell death. Oncotarget 2017, 8, 67254–67268. [Google Scholar] [CrossRef]
- Ovsjanko, E.V.; Efremov, A.V.; Michurina, S.V.; Bgatova, N.P.; Lushnikova, E.L.; Ovsjanko, Y.U.; Vakulin, G.M. Ultrastructural and stereological analysis of walker 256 carcinosarcoma cells at various stages of their differentiation. Bull. Exp. Biol. Med. 2009, 48, 447–451. [Google Scholar] [CrossRef]
- Yang, K.; Huang, C.; Chi, M.; Chiang, H.; Wang, Y.; Hsia, C.; Andocs, G.; Wang, H.; Chi, K. In vitro comparison of conventional hyperthermia and modulated electro-hyperthermia. Oncotarget 2016, 7, 84082–84092. [Google Scholar] [CrossRef] [Green Version]
- Orlandi, L.; Zaffaroni, N.; Bearzatto, A.; Costa, A.; Supino, R.; Vaglini, M.; Silvestrini, R. Effect of melphalan and hyperthermia on cell cycle progression and cyclin B1 expression in human melanoma cells. Cell Prolif. 1995, 28, 617–630. [Google Scholar] [CrossRef]
- Abe, T.; Tamiya, T.; Ono, Y.; Salker, A.H.; Akiyama, K.; Ohmoto, T. Accumulation of cell cycle regulatory proteins, p21 and p27, induced after hyperthermia in human glioma cells. Int. J. Hyperth. 2001, 17, 499–507. [Google Scholar]
- Gertz, F.; Azimov, R.; Khitun, A. Biological cell positioning and spatially selective destruction via magnetic nanoparticles. Appl. Phys. Lett. 2012, 101, 013701. [Google Scholar] [CrossRef]
- Xie, J.; Yan, C.; Yan, Y.; Chen, L.; Song, L.; Zang, F.; An, Y.; Teng, G.; Gu, N.; Zhang, Y. Multi-modal Mn–Zn ferrite nanocrystals for magnetically-induced cancer targeted hyperthermia: A comparison of passive and active targeting effects. Nanoscale 2016, 8, 16902–16915. [Google Scholar] [CrossRef] [PubMed]
- Elkhova, T.M.; Gun’ko, Y.K.; Pyatakov, A.P.; Spichkin, Y.I.; Kenneth, D.; Tishin, A.M. The expeimental setup for measuring of thermal parameters of magnetic fluids in AC magnetic field. Solid State Phenom. 2014, 215, 454–458. [Google Scholar] [CrossRef]
- Pimentel, B.; Caraballo-Vivas, R.J.; Checca, N.R.; Zverev, V.I.; Salakhova, R.T.; Makarova, L.A.; Pyatakov, A.P.; Perov, N.S.; Tishin, A.M.; Shtil, A.A.; et al. Threshold heating temperature for magnetic hyperthermia: Controlling the heat exchange with the blocking temperature of magnetic nanoparticles. J. Solid State Chem. 2018, 260, 34–38. [Google Scholar] [CrossRef]
- Habriev, R.U. Guideline for Experimental (Preclinical) Studying of New Pharmacological Substances, 2nd ed.; Medicine: Moscow, Russia, 2005; pp. 41–54. [Google Scholar]
- Rybakova, A.V.; Makarova, M.N.; Kukharenko, A.E.; Vichare, A.S.; Rueffer, F.-R. Current Requirements for and Approaches to Dosing in Animal Studies. Bull. Sci. Cent. Expert Eval. Med. Prod. 2018, 8, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, L.; Shi, Y.; Javidiparsijani, S.; Wang, G.; Li, X.; Ouyang, W.; Zhou, J.; Zhao, L.; Wang, X.; et al. Abscopal antitumor immune effects of magnet-mediated hyperthermia at a high therapeutic temperature on Walker-256 carcinosarcomas in rats. Oncol. Lett. 2014, 7, 764–770. [Google Scholar] [CrossRef]
- Tripodi, A.A.P.; Randelovic, I.; Biri-Kovacs, B.; Szeder, B.; Mezo, G.; Tovari, J. In Vivo Tumor Growth Inhibition and Antiangiogenic Effect of Cyclic NGR Peptide-Daunorubicin Conjugates Developed for Targeted Drug Delivery. Pathol. Oncol. Res. 2020, 26, 1879–1892. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8, 2082. [Google Scholar] [CrossRef]






| Parameter | Dose, mg Fe/kg | 95% Confidence Intervals Limits | |
|---|---|---|---|
| Lower | Upper | ||
| LD16 | 183.8 | 20.6 | 347.4 |
| LD50 | 652.3 | 343.2 | 1044.8 |
| LD84 | 2315.5 | 1340.3 | 8488.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulikov, O.A.; Zharkov, M.N.; Ageev, V.P.; Yakobson, D.E.; Shlyapkina, V.I.; Zaborovskiy, A.V.; Inchina, V.I.; Balykova, L.A.; Tishin, A.M.; Sukhorukov, G.B.; et al. Magnetic Hyperthermia Nanoarchitectonics via Iron Oxide Nanoparticles Stabilised by Oleic Acid: Anti-Tumour Efficiency and Safety Evaluation in Animals with Transplanted Carcinoma. Int. J. Mol. Sci. 2022, 23, 4234. https://doi.org/10.3390/ijms23084234
Kulikov OA, Zharkov MN, Ageev VP, Yakobson DE, Shlyapkina VI, Zaborovskiy AV, Inchina VI, Balykova LA, Tishin AM, Sukhorukov GB, et al. Magnetic Hyperthermia Nanoarchitectonics via Iron Oxide Nanoparticles Stabilised by Oleic Acid: Anti-Tumour Efficiency and Safety Evaluation in Animals with Transplanted Carcinoma. International Journal of Molecular Sciences. 2022; 23(8):4234. https://doi.org/10.3390/ijms23084234
Chicago/Turabian StyleKulikov, Oleg A., Mikhail N. Zharkov, Valentin P. Ageev, Denis E. Yakobson, Vasilisa I. Shlyapkina, Andrey V. Zaborovskiy, Vera I. Inchina, Larisa A. Balykova, Alexander M. Tishin, Gleb B. Sukhorukov, and et al. 2022. "Magnetic Hyperthermia Nanoarchitectonics via Iron Oxide Nanoparticles Stabilised by Oleic Acid: Anti-Tumour Efficiency and Safety Evaluation in Animals with Transplanted Carcinoma" International Journal of Molecular Sciences 23, no. 8: 4234. https://doi.org/10.3390/ijms23084234