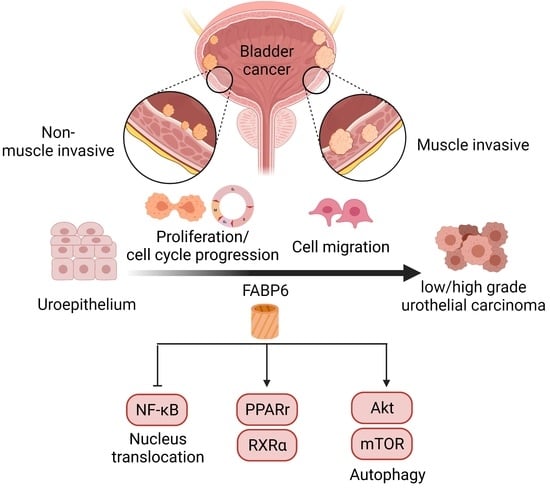
Fatty Acid Binding Protein 6 Inhibition Decreases Cell Cycle Progression, Migration and Autophagy in Bladder Cancers
Abstract
:1. Introduction
2. Results
2.1. Expression Pattern of FABP6 in Uroepithelium, Low- and High-Grade Urothelial Carcinomas
2.2. Knockdown of FABP6 Inhibited Survival and Colony Formation in BC Cells
2.3. Knockdown of FABP6 induced Cell Cycle Arrest of BC Cells
2.4. Reducing of FABP6 Inhibited Autophagy Markers via Akt/mTOR Pathway
2.5. FABP6 Inhibition Decreased Nuclear Receptor PPARγ/RXRα and Activated NF-kB Signaling
2.6. Reducing of FABP6 Decreased Cell Migration and Invasion Characteristics in siFABP6-Transfected TSGH-8301 and T24 Cells
2.7. FABP6-Knockdown Suppressed BC Cell Growth In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Small Interfering RNA (siRNA) Transfection
4.3. Short Hairpin RNA (shRNA) Transfection
4.4. Lentivirus Production
4.5. MTT Assays
4.6. Flow Cytometric Cell-Cycle Analysis
4.7. Western Blotting
4.8. Wound Healing Scratch Assay
4.9. Transwell Assays
4.10. Xenograft Mouse Model
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richters, A.; Aben, K.K.H.; Kiemeney, L. The global burden of urinary bladder cancer: An update. World J. Urol. 2020, 38, 1895–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.S.; Lara, P.N., Jr.; Pan, C.X. Progress in personalizing chemotherapy for bladder cancer. Adv. Urol. 2012, 2012, 364919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.T.; Huillard, O.; Dabi, Y.; Anract, J.; Sibony, M.; Zerbib, M.; Xylinas, E. Neoadjuvant Chemotherapy in Patients with Muscle-Invasive Bladder Cancer and Its Impact on Surgical Morbidity and Oncological Outcomes: A Real-World Experience. Front. Surg. 2018, 5, 58. [Google Scholar] [CrossRef] [Green Version]
- Malmstrom, P.U.; Rintala, E.; Wahlqvist, R.; Hellstrom, P.; Hellsten, S.; Hannisdal, E. Five-year followup of a prospective trial of radical cystectomy and neoadjuvant chemotherapy: Nordic Cystectomy Trial I. The Nordic Cooperative Bladder Cancer Study Group. J. Urol. 1996, 155, 1903–1906. [Google Scholar] [CrossRef]
- Amiri, M.; Yousefnia, S.; Seyed Forootan, F.; Peymani, M.; Ghaedi, K.; Nasr Esfahani, M.H. Diverse roles of fatty acid binding proteins (FABPs) in development and pathogenesis of cancers. Gene 2018, 676, 171–183. [Google Scholar] [CrossRef]
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008, 7, 489–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohmachi, T.; Inoue, H.; Mimori, K.; Tanaka, F.; Sasaki, A.; Kanda, T.; Fujii, H.; Yanaga, K.; Mori, M. Fatty acid binding protein 6 is overexpressed in colorectal cancer. Clin. Cancer Res. 2006, 12, 5090–5095. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Song, H.; Zeng, X.; Quan, M.; Gao, Y. Screening and discrimination of optimal prognostic genes for pancreatic cancer based on a prognostic prediction model. G3 2021, 11, jkab296. [Google Scholar] [CrossRef]
- Doherty, S.C.; McKeown, S.R.; McKelvey-Martin, V.; Downes, C.S.; Atala, A.; Yoo, J.J.; Simpson, D.A.; Kaufmann, W.K. Cell cycle checkpoint function in bladder cancer. J. Natl. Cancer Inst. 2003, 95, 1859–1868. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef] [Green Version]
- Hoo, R.L.; Shu, L.; Cheng, K.K.; Wu, X.; Liao, B.; Wu, D.; Zhou, Z.; Xu, A. Adipocyte Fatty Acid Binding Protein Potentiates Toxic Lipids-Induced Endoplasmic Reticulum Stress in Macrophages via Inhibition of Janus Kinase 2-dependent Autophagy. Sci. Rep. 2017, 7, 40657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.G.; Jung, K.H.; Lee, D.G.; Yoon, J.H.; Choi, K.S.; Kwon, S.W.; Shen, H.M.; Morgan, M.J.; Hong, S.S.; Kim, Y.S. 20(S)-Ginsenoside Rg3 is a novel inhibitor of autophagy and sensitizes hepatocellular carcinoma to doxorubicin. Oncotarget 2014, 5, 4438–4451. [Google Scholar] [CrossRef] [Green Version]
- Soto-Avellaneda, A.; Morrison, B.E. Signaling and other functions of lipids in autophagy: A review. Lipids Health Dis. 2020, 19, 214. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, W.J.; Lee, S.W.; Chae, H.W.; Kim, D.H.; Oh, Y. Insulin-like growth factor binding protein-3 induces G1 cell cycle arrest with inhibition of cyclin-dependent kinase 2 and 4 in MCF-7 human breast cancer cells. Horm. Metab. Res. 2010, 42, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Bartkova, J.; Lukas, J.; Strauss, M.; Bartek, J. Cyclin D3: Requirement for G1/S transition and high abundance in quiescent tissues suggest a dual role in proliferation and differentiation. Oncogene 1998, 17, 1027–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, Y.; Huang, S.; Liu, M.; Guo, L.; Shen, X.; Wu, J. Cyclin D3 predicts disease-free survival in breast cancer. Cancer Cell Int. 2015, 15, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunz, F.; Dutriaux, A.; Lengauer, C.; Waldman, T.; Zhou, S.; Brown, J.P.; Sedivy, J.M.; Kinzler, K.W.; Vogelstein, B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 1998, 282, 1497–1501. [Google Scholar] [CrossRef]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef] [PubMed]
- Hojman, P.; Fjelbye, J.; Zerahn, B.; Christensen, J.F.; Dethlefsen, C.; Lonkvist, C.K.; Brandt, C.; Gissel, H.; Pedersen, B.K.; Gehl, J. Voluntary exercise prevents cisplatin-induced muscle wasting during chemotherapy in mice. PLoS ONE 2014, 9, e109030. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Dong, C.; Zhang, J.; Su, L.; Wang, X.; Cui, H.; Chen, Z. Combination therapy of anti-cancer bioactive peptide with Cisplatin decreases chemotherapy dosing and toxicity to improve the quality of life in xenograft nude mice bearing human gastric cancer. Cell Biosci. 2014, 4, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Yang, X.; Tang, J.; Si, S.; Zhou, Z.; Lu, J.; Han, J.; Yuan, B.; Wu, Q.; Lu, Q. ALKBH5 inhibited cell proliferation and sensitized bladder cancer cells to cisplatin by m6A-CK2α-mediated glycolysis. Mol. Ther. Nucleic Acids 2021, 23, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Chen, L.-w.; Chen, Y.-C.; Chan, S.-T.; Liao, J.-W.; Yeh, S.-L. Quercetin attenuates cisplatin-induced fat loss. Eur. J. Nutr. 2021, 60, 1781–1793. [Google Scholar] [CrossRef] [PubMed]
- Rupp, T.; Pelouin, O.; Genest, L.; Legrand, C.; Froget, G.; Castagne, V. Therapeutic potential of Fingolimod in triple negative breast cancer preclinical models. Transl. Oncol. 2021, 14, 100926. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Yang, J.; Li, G.; You, Q.; Han, W.; Li, T.; Gao, D.; Xie, X.; Lee, B.H.; Du, J.; et al. Ubiquitylation of p62/sequestosome1 activates its autophagy receptor function and controls selective autophagy upon ubiquitin stress. Cell Res. 2017, 27, 657–674. [Google Scholar] [CrossRef]
- Yoshida, G.J. Therapeutic strategies of drug repositioning targeting autophagy to induce cancer cell death: From pathophysiology to treatment. J. Hematol. Oncol. 2017, 10, 67. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; He, C.; Ye, F.; Ye, E.; He, H.; Chen, G.; Zhang, J. p62 Overexpression Promotes Bone Metastasis of Lung Adenocarcinoma out of LC3-Dependent Autophagy. Front. Oncol. 2021, 11, 609548. [Google Scholar] [CrossRef]
- Paquette, M.; El-Houjeiri, L.; Pause, A. mTOR pathways in cancer and autophagy. Cancers 2018, 10, 18. [Google Scholar] [CrossRef] [Green Version]
- Kondo, Y.; Kondo, S. Autophagy and cancer therapy. Autophagy 2006, 2, 85–90. [Google Scholar] [CrossRef]
- Cha-Molstad, H.; Yu, J.E.; Feng, Z.; Lee, S.H.; Kim, J.G.; Yang, P.; Han, B.; Sung, K.W.; Yoo, Y.D.; Hwang, J. p62/SQSTM1/Sequestosome-1 is an N-recognin of the N-end rule pathway which modulates autophagosome biogenesis. Nat. Commun. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Li, F.; Guo, H.; Yang, Y.; Feng, M.; Liu, B.; Ren, X.; Zhou, H. Autophagy modulation in bladder cancer development and treatment. Oncol. Rep. 2019, 42, 1647–1655. [Google Scholar] [CrossRef]
- Neels, J.G.; Grimaldi, P.A. Physiological functions of peroxisome proliferator-activated receptor beta. Physiol. Rev. 2014, 94, 795–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta 2011, 1812, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Schachtrup, C.; Emmler, T.; Bleck, B.; Sandqvist, A.; Spener, F. Functional analysis of peroxisome-proliferator-responsive element motifs in genes of fatty acid-binding proteins. Biochem. J. 2004, 382, 239–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfrum, C.; Borrmann, C.M.; Borchers, T.; Spener, F. Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors alpha—and gamma-mediated gene expression via liver fatty acid binding protein: A signaling path to the nucleus. Proc. Natl. Acad. Sci. USA 2001, 98, 2323–2328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garin-Shkolnik, T.; Rudich, A.; Hotamisligil, G.S.; Rubinstein, M. FABP4 attenuates PPARgamma and adipogenesis and is inversely correlated with PPARgamma in adipose tissues. Diabetes 2014, 63, 900–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, T.N.M.; Kim, M.K.; Vo, V.T.A.; Choi, J.W.; Choi, J.H.; Kim, H.W.; Cha, S.K.; Park, K.S.; Jeong, Y. Inhibition of oncogenic Src induces FABP4-mediated lipolysis via PPARgamma activation exerting cancer growth suppression. EBioMedicine 2019, 41, 134–145. [Google Scholar] [CrossRef] [Green Version]
- Bo, Q.L.; Chen, Y.H.; Yu, Z.; Fu, L.; Zhou, Y.; Zhang, G.B.; Wang, H.; Zhang, Z.H.; Xu, D.X. Rosiglitazone pretreatment protects against lipopolysaccharide-induced fetal demise through inhibiting placental inflammation. Mol. Cell Endocrinol. 2016, 423, 51–59. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-H.; Chang, H.-H.; Lai, C.-R.; Wang, H.-H.; Tsai, W.-C.; Tsai, Y.-L.; Changchien, C.-Y.; Cheng, Y.-C.; Wu, S.-T.; Chen, Y. Fatty Acid Binding Protein 6 Inhibition Decreases Cell Cycle Progression, Migration and Autophagy in Bladder Cancers. Int. J. Mol. Sci. 2022, 23, 2154. https://doi.org/10.3390/ijms23042154
Lin C-H, Chang H-H, Lai C-R, Wang H-H, Tsai W-C, Tsai Y-L, Changchien C-Y, Cheng Y-C, Wu S-T, Chen Y. Fatty Acid Binding Protein 6 Inhibition Decreases Cell Cycle Progression, Migration and Autophagy in Bladder Cancers. International Journal of Molecular Sciences. 2022; 23(4):2154. https://doi.org/10.3390/ijms23042154
Chicago/Turabian StyleLin, Chieh-Hsin, Hsin-Han Chang, Chien-Rui Lai, Hisao-Hsien Wang, Wen-Chiuan Tsai, Yu-Ling Tsai, Chih-Ying Changchien, Yu-Chen Cheng, Sheng-Tang Wu, and Ying Chen. 2022. "Fatty Acid Binding Protein 6 Inhibition Decreases Cell Cycle Progression, Migration and Autophagy in Bladder Cancers" International Journal of Molecular Sciences 23, no. 4: 2154. https://doi.org/10.3390/ijms23042154