The Consequences of Water Interactions with Nitrogen-Containing Carbonaceous Quantum Dots—The Mechanistic Studies
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. The N-CQDs Synthesis
3.2. N-CQDs Physicochemical Characterization
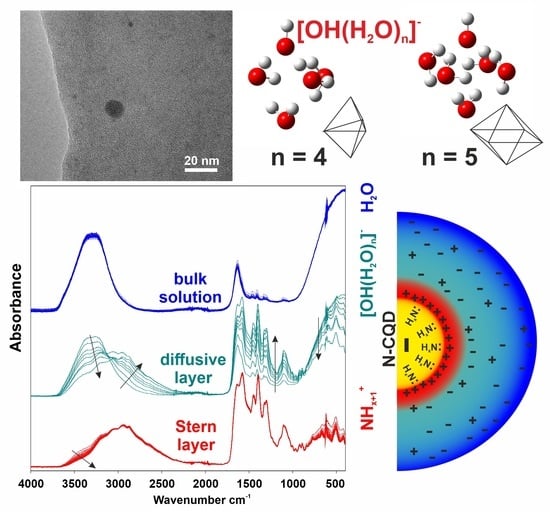
3.3. ATR FTIR Analysis
3.4. Calorimetric Analysis
3.5. Ab Initio Studies
4. Conclusions
- (i)
- the dissociative H2O chemisorption on superhydrophilic surfaces, for which 0° WCA, occurs in a low-pressure range;
- (ii)
- effective protolysis causes the formation of the [OH(H2O)n]− water clusters arising during this process;
- (iii)
- their size (n) determines the conductivity;
- (iv)
- formed thus, Eigen-like water complexes appear in the diffusive and Stern layers and create a dense, stable, and nonconductive layer close to the N-CQDs surface;
- (v)
- proposed here, the “evaporating droplet ATR technique” is perfect for the characterization of materials soluble in water.
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Carbon quantum dots from natural resource: A review. Mat. Today Chem. 2018, 8, 96–109. [Google Scholar] [CrossRef]
- Pourreza, N.; Ghomi, M. Green synthesized carbon quantum dots from Prosopis juliflora leaves as a dual off-on fluorescence probe for sensing mercury (II) and chemet drug. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Travas-Sejdic, J. Simple aqueous solution route to luminescent carbogenic dots from car-bohydrates. Chem. Mater. 2009, 21, 5563–5565. [Google Scholar] [CrossRef]
- Liang, Q.; Ma, W.; Shi, Y.; Li, Z.; Yang, X. Easy synthesis of highly fluorescent carbon quantum dots from gelatine and their luminescent properties and applications. Carbon 2013, 60, 421–428. [Google Scholar] [CrossRef]
- Zhang, Z.; Hao, J.; Zhang, J.; Zhang, B.; Tang, J. Protein as the source for synthesizing fluorescent carbon dots by a one-pot hydrothermal route. RSC Adv. 2012, 2, 8599–8601. [Google Scholar] [CrossRef]
- Zhu, C.; Zhai, J.; Dong, S. Bifunctional fluorescent carbon nanodots: Green synthesis via soy milk and application as metal-free electrocatalysts for oxygen reduction. Chem. Commun. 2012, 48, 9367–9369. [Google Scholar] [CrossRef]
- Gu, J.; Wang, W.; Zhang, Q.; Meng, Z.; Jia, X.; Xi, K. Synthesis of fluorescent carbon nanoparticles from polyacrylamide for fast cellular endocytosis. RSC Adv. 2013, 3, 15589–15591. [Google Scholar] [CrossRef]
- Wiśniewski, M.; Czarnecka, J.; Bolibok, P.; Świdziński, M.; Roszek, K. New insight into the fluorescence quenching of nitrogen-containing carbonaceous quantum dots—From surface chemistry to biomedical applications. Materials 2021, 14, 2454. [Google Scholar] [CrossRef]
- Luo, P.G.; Yang, F.; Yang, S.-T.; Sonkar, S.K.; Yang, L.; Broglie, J.J.; Liua, Y.; Sun, Y.-P. Carbon-based quantum dots for fluorescence imaging of cells and tissues. RSC Adv. 2014, 4, 10791–10807. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Itzhaki, E.; Hadad, E.; Gedanken, A.; Margel, S. Microwave-Synthesized Polysaccharide-derived carbon dots as therapeutic cargoes and toughening agents for elastomeric gels. ACS Appl. Mater. Interfaces 2020, 12, 51940–51951. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Margel, S.; Gedanken, A. Tailor made magnetic nanolights: Fabrication to cancer theranostics applications. Nanoscale Adv. 2021, 3, 6762–6796. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Kaur, M.; Sharma, V.K. Nitrogen-doped graphene and graphene quantum dots: A review onsynthesis and applications in energy, sensors and environment. Adv. Coll. Int. Sci. 2018, 259, 44–64. [Google Scholar] [CrossRef]
- Tyrode, E.; Sengupta, E.; Sthoer, A. Identifying eigen-like hydrated protons at negatively charged interfaces. Nat. Comm. 2020, 11, 493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Headrick, J.M.; Diken, E.G.; Walters, R.S.; Hammer, N.I.; Christie, R.A.; Cui, J.; Myshakin, E.M.; Duncan, M.A.; Johnson, M.A.; Jordan, K.D. Spectral Signatures of Hydrated Proton Vibrations in Water Clusters. Science 2005, 308, 1765–1769. [Google Scholar] [CrossRef] [PubMed]
- Thämer, M.; De Marco, L.; Ramasesha, K.; Mandal, A.; Tokmakoff, A. Ultrafast 2D IR spectroscopy of the excess proton in liquid water. Science 2015, 350, 78–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichii, T.; Arikawa, T.; Omoto, K.; Hosono, N.; Sato, H.; Kitagawa, S.; Tanaka, K. Observation of an exotic state of water in the hydrophilic nanospace of porous coordination polymers. Comm. Chem. 2020, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Pearson, R.G. Acids and bases. Science 1966, 151, 172–177. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512. [Google Scholar] [CrossRef]
- Alfarra, A.; Frackowiak, E.; Beguin, F. The HSAB concept as a means to interpret the adsorption of metal ions onto activated carbons. Appl. Surf. Sci. 2004, 228, 84–92. [Google Scholar] [CrossRef]
- Wisniewski, M.; Gauden, P.A. Pearson’s Hard–Soft Acid–Base Principle as a Means of interpreting the reactivity of carbon materials. Ads. Sci. Tech. 2006, 24, 389–402. [Google Scholar] [CrossRef]
- Saito, M.; Kawaharasaki, S.; Ito, K.; Yamada, S.; Hayamizub, K.; Seki, S. Strategies for fast ion transport in electrochemical capacitor electrolytes from diffusion coefficients, ionic conductivity, viscosity, density and interaction energies based on HSAB theory. RSC Adv. 2017, 7, 14528–14535. [Google Scholar] [CrossRef] [Green Version]
- Dordevic, L.; Arcudi, F.; Cacioppo, M.; Prato, M. A multifunctional chemical toolbox to engineer carbon dots for biomedical and energy applications. Nat. Nanotechnol. 2022, 17, 112–130. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, A.; Bibi, N.; Hyder, S.; Muhammad, A.; Ren, H.; Liu, J.; Lei, Z. Recent advances in green carbon dots (2015–2022): Synthesis, metal ion sensing, and biological applications. Beilstein J. Nanotechnol. 2022, 13, 1068–1107. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Maruthapandi, M.; Das, P.; Ganguly, S.; Margel, S.; Luong, J.H.T.; Gedanken, A. Applications of N-doped carbon dots as antimicrobial agents, antibiotic carriers, and selective fluorescent probes for nitro explosives. ACS Appl. Bio Mater. 2020, 3, 8023–8031. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Zhao, S.; Wang, B.; Song, X.; Xiao, J.; Lana, M. Synthesis strategies, luminescence mechanisms, and biomedical applications of near-infrared fluorescent carbon dots. Coord. Chem. Rev. 2022, 470, 214703. [Google Scholar] [CrossRef]
- Tahira, R.; Sameera, S.; Farooq, S. Smart nano-architectures as potential sensing tools for detecting heavy metal ions in aqueous matrices. Trends in Environmental Anal. Chem. 2022, 36, e00179. [Google Scholar]
- Czarnecka, J.; Kwiatkowski, M.; Wiśniewski, M.; Roszek, K. Protein corona hinders N-CQDs oxidative potential and favors their application as nanobiocatalytic system. Int. J. Mol. Sci. 2021, 22, 8136. [Google Scholar] [CrossRef]
- D’Arcy, R.L.; Watt, I.C. Analysis of sorption isotherms of non-homogeneous sorbents. Trans Faraday Soc. 1970, 66, 1236–1245. [Google Scholar] [CrossRef]
- Kim, I.; Jens, C.M.; Grimstvedt, A.; Svendsen, H.F. Thermodynamics of protonation of amines in aqueous solutions at elevated temperatures. J. Chem. Thermodynamics. 2011, 43, 1754–1762. [Google Scholar] [CrossRef]
- Agmon, N.; Bakker, H.J.; Kramer Campen, R.; Henchman, R.H.; Pohl, P.; Roke, S.; Thämer, M.; Hassanali, A. Protons and Hydroxide Ions in Aqueous Systems. Chem. Rev. 2016, 116, 7642–7672. [Google Scholar] [CrossRef]
- Gonella, G.; Backus, E.H.G.; Nagata, Y.; Bonthuis, D.J.; Loche, P.; Schlaich, A.; Netz, R.R.; Kühnle, A.; McCrum, I.T.; Koper, M.T.M.; et al. Water at charged interfaces. Nature Rev. Chem. 2021, 5, 466–485. [Google Scholar] [CrossRef]
- Lv, H.; Gao, X.; Xu, Q.; Liu, H.; Wang, Y.-G.; Xia, Y. Carbon Quantum Dot-Induced MnO2 Nanowire Formation and Construction of a Binder-Free Flexible Membrane with Excellent Superhydrophilicity and Enhanced Supercapacitor Performance. ACS Appl. Mater. Interfaces. 2017, 9, 40394–40403. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Wu, L.; Lu, Z.; Li, M.; Wen, Y.; Yang, Y.; Liu, W.; Zhang, T.; Cao, W.; Tsang, S.-W.; et al. High efficiency and stability of ink-jet printed quantum dot light emitting diodes. Nat. Comm. 2020, 11, 1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Gillespie, S.E.; Oscarson, J.L.; Izatt, R.M. Enthalpy of dissociation of water at 325 °C and LogK, ΔH, ΔS, and ΔCp values for the formation of NaOH(aq) from 250 to 325 °C. J. Sol. Chem. 1992, 21, 803–824. [Google Scholar] [CrossRef]
- Sebben, D.; Pendleton, P. Infrared spectrum analysis of the dissociated states of simple amino acids. Spectrochim. Acta Mol. Biomol. Spectrosc. 2014, 132, 706–712. [Google Scholar] [CrossRef]
- Zawadzki, J.; Wiśniewski, M.; Skowrońska, K. Heterogeneous Reactions of NO2 and NO-O2 on the Surface of Carbons. Carbon 2003, 41, 235–246. [Google Scholar] [CrossRef]
- Wisniewski, M.; Rychlicki, G.; Arcimowicz, A. Experimental and theoretical estimations of the polar force contributions to the heat of immersion of carbon nanotubes. Chem. Phys. Lett. 2010, 485, 331. [Google Scholar] [CrossRef]
- Frisch, M.J. Gaussian ’09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Terzyk, A.P.; Furmaniak, S.; Wiśniewski, M.; Werengowska, K.; Gauden, P.A.; Kowalczyk, P. New findings on the influence of carbon surface curvature on energetics of benzene adsorption from gaseous phase. Chem. Phys. Lett. 2016, 645, 157–163. [Google Scholar] [CrossRef]
- Furmaniak, S.; Wiśniewski, M.; Werengowska-Ciećwierz, K.; Terzyk, A.P.; Hata, K.; Gauden, P.A.; Kowalczyk, P.; Szybowicz, M. Water at Curved Carbon Surface: Mechanisms of Adsorption Revealed by First Calorimetric Study. J. Phys. Chem. C 2015, 119, 2703–2715. [Google Scholar] [CrossRef]
- Koopmans, T.A. Uber die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica 1933, 1, 104–113. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiśniewski, M. The Consequences of Water Interactions with Nitrogen-Containing Carbonaceous Quantum Dots—The Mechanistic Studies. Int. J. Mol. Sci. 2022, 23, 14292. https://doi.org/10.3390/ijms232214292
Wiśniewski M. The Consequences of Water Interactions with Nitrogen-Containing Carbonaceous Quantum Dots—The Mechanistic Studies. International Journal of Molecular Sciences. 2022; 23(22):14292. https://doi.org/10.3390/ijms232214292
Chicago/Turabian StyleWiśniewski, Marek. 2022. "The Consequences of Water Interactions with Nitrogen-Containing Carbonaceous Quantum Dots—The Mechanistic Studies" International Journal of Molecular Sciences 23, no. 22: 14292. https://doi.org/10.3390/ijms232214292