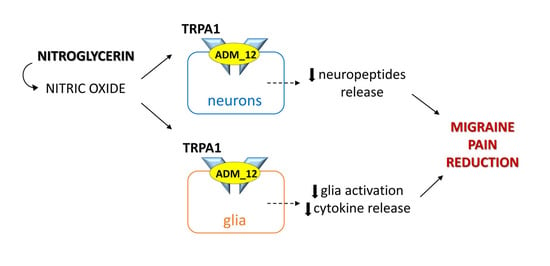
Modulation of Glia Activation by TRPA1 Antagonism in Preclinical Models of Migraine
Abstract
:1. Introduction
2. Results
2.1. ADM_12 Counteracts Nitroglycerin (NTG)-Induced Hyperalgesia
2.2. ADM_12 Effects on CGRP Gene and Protein Expression in TG
2.3. ADM_12 Reduced NTG-Induced Neuroinflammation at Central and Peripheral Levels
2.3.1. Effects on Inflammation-Related Glial Activation and Macrophage Cell Infiltration
2.3.2. Effects of ADM_12 on Pro-Inflammatory and Anti-Inflammatory Markers
3. Discussion
3.1. TRPA1 Antagonism in Migraine-like Pain
3.2. TRPA1 Antagonism and Neuroinflammation
3.3. Limitations of the Study and Future Directions
4. Materials and Methods
4.1. Animals
4.2. Experimental Plan
4.3. Orofacial Formalin Test
4.4. Immunofluorescence Staining
4.5. Image Analysis
4.6. Real-Time PCR
4.7. Statistical Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef] [Green Version]
- Olesen, J. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Ferrari, M.D.; Goadsby, P.J.; Burstein, R.; Kurth, T.; Ayata, C.; Charles, A.; Ashina, M.; van den Maagdenberg, A.; Dodick, D.W. Migraine. Nat. Rev. Dis. Primers 2022, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Noseda, R.; Burstein, R. Migraine pathophysiology: Anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain 2013, 154 (Suppl. S1), S44–S53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashina, M.; Hansen, J.M.; Do, T.P.; Melo-Carrillo, A.; Burstein, R.; Moskowitz, M.A. Migraine and the trigeminovascular system-40 years and counting. Lancet Neurol. 2019, 18, 795–804. [Google Scholar] [CrossRef]
- Biscetti, L.; De Vanna, G.; Cresta, E.; Bellotti, A.; Corbelli, I.; Cupini, M.L.; Calabresi, P.; Sarchielli, P. Immunological findings in patients with migraine and other primary headaches: A narrative review. Clin. Exp. Immunol. 2022, 207, 11–26. [Google Scholar] [CrossRef]
- Hanani, M.; Spray, D.C. Emerging importance of satellite glia in nervous system function and dysfunction. Nat. Rev. Neurosci. 2020, 21, 485–498. [Google Scholar] [CrossRef]
- Grace, P.M.; Tawfik, V.L.; Svensson, C.I.; Burton, M.D.; Loggia, M.L.; Hutchinson, M.R. The Neuroimmunology of Chronic Pain: From Rodents to Humans. J. Neurosci. 2021, 41, 855–865. [Google Scholar] [CrossRef]
- Wieseler-Frank, J.; Maier, S.; Watkins, L. Glial activation and pathological pain. Neurochem. Int. 2004, 45, 389–395. [Google Scholar] [CrossRef]
- Milligan, E.D.; Watkins, L.R. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 2009, 10, 23–36. [Google Scholar] [CrossRef] [Green Version]
- Cavestro, C.; Ferrero, M.; Mandrino, S.; Di Tavi, M.; Rota, E. Novelty in Inflammation and Immunomodulation in Migraine. Curr. Pharm. Des. 2019, 25, 2919–2936. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.S.; Mainero, C.; Ichijo, E.; Ward, N.; Granziera, C.; Zürcher, N.R.; Akeju, O.; Bonnier, G.; Price, J.; Hooker, J.M.; et al. Imaging of neuroinflammation in migraine with aura: A [11C]PBR28 PET/MRI study. Neurology 2019, 92, e2038–e2050. [Google Scholar] [CrossRef] [PubMed]
- Bruno, P.P.; Carpino, F.; Carpino, G.; Zicari, A. An overview on immune system and migraine. Eur. Rev. Med. Pharmacol. Sci. 2007, 11, 245–248. [Google Scholar] [PubMed]
- Conti, P.; D’Ovidio, C.; Conti, C.; Gallenga, C.E.; Lauritano, D.; Caraffa, A.; Kritas, S.K.; Ronconi, G. Progression in migraine: Role of mast cells and pro-inflammatory and anti-inflammatory cytokines. Eur. J. Pharmacol. 2019, 844, 87–94. [Google Scholar] [CrossRef]
- Malick, A.; Burstein, R. Peripheral and central sensitization during migraine. Funct. Neurol. 2000, 15, S28–S35. [Google Scholar]
- Demartini, C.; Tassorelli, C.; Zanaboni, A.M.; Tonsi, G.; Francesconi, O.; Nativi, C.; Greco, R. The role of the transient receptor potential ankyrin type-1 (TRPA1) channel in migraine pain: Evaluation in an animal model. J. Headache Pain 2017, 18, 94. [Google Scholar] [CrossRef]
- Marone, I.M.; De Logu, F.; Nassini, R.; De Carvalho Goncalves, M.; Benemei, S.; Ferreira, J.; Jain, P.; Li Puma, S.; Bunnett, N.W.; Geppetti, P.; et al. TRPA1/NOX in the soma of trigeminal ganglion neurons mediates migraine-related pain of glyceryl trinitrate in mice. Brain 2018, 141, 2312–2328. [Google Scholar] [CrossRef] [Green Version]
- Christensen, S.L.; Rasmussen, R.H.; Ernstsen, C.; La Cour, S.; David, A.; Chaker, J.; Haanes, K.A.; Christensen, S.T.; Olesen, J.; Kristensen, D.M. CGRP-dependent signalling pathways involved in mouse models of GTN- cilostazol- and levcromakalim-induced migraine. Cephalalgia 2021, 41, 1413–1426. [Google Scholar] [CrossRef]
- Kim, S.-J.; Yeo, J.-H.; Yoon, S.-Y.; Roh, D.-H. Different Involvement of ASIC and TRPA1 in Facial and Hindpaw Allodynia in Nitroglycerin-Induced Peripheral Hypersensitivities in Mice. Life 2022, 12, 1294. [Google Scholar] [CrossRef]
- Benemei, S.; Fusi, C.; Trevisan, G.; Geppetti, P. The TRPA1 channel in migraine mechanism and treatment. Br. J. Pharmacol. 2014, 171, 2552–2567. [Google Scholar] [CrossRef] [Green Version]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef]
- Eberhardt, M.; Dux, M.; Namer, B.; Miljkovic, J.; Cordasic, N.; Will, C.; Kichko, T.I.; de la Roche, J.; Fischer, M.; Suárez, S.A.; et al. H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. Nat. Commun. 2014, 5, 4381. [Google Scholar] [CrossRef] [Green Version]
- Diogenes, A.; Akopian, A.N.; Hargreaves, K.M. NGF up-regulates TRPA1: Implications for orofacial pain. J. Dent. Res. 2007, 86, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Hatano, N.; Itoh, Y.; Suzuki, H.; Muraki, Y.; Hayashi, H.; Onozaki, K.; Wood, I.C.; Beech, D.J.; Muraki, K. Hypoxia-inducible factor-1α (HIF1α) switches on transient receptor potential ankyrin repeat 1 (TRPA1) gene expression via a hypoxia response element-like motif to modulate cytokine release. J. Biol. Chem. 2012, 287, 31962–31972. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.M.; Itson-Zoske, B.; Cai, Y.; Qiu, C.; Pan, B.; Stucky, C.L.; Hogan, Q.H.; Yu, H. Satellite glial cells in sensory ganglia express functional transient receptor potential ankyrin 1 that is sensitized in neuropathic and inflammatory pain. Mol. Pain 2020, 16, 1744806920925425. [Google Scholar] [CrossRef] [PubMed]
- De Logu, F.; Nassini, R.; Hegron, A.; Landini, L.; Jensen, D.D.; Latorre, R.; Ding, J.; Marini, M.; de Araujo, D.S.M.; Ramírez-Garcia, P.; et al. Schwann cell endosome CGRP signals elicit periorbital mechanical allodynia in mice. Nat. Commun. 2022, 13, 646. [Google Scholar] [CrossRef] [PubMed]
- Shigetomi, E.; Tong, X.; Kwan, K.Y.; Corey, D.P.; Khakh, B.S. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat. Neurosci. 2011, 15, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Billeter, A.T.; Galbraith, N.; Walker, S.; Lawson, C.; Gardner, S.A.; Sarojini, H.; Galandiuk, S.; Polk, H.C., Jr. TRPA1 mediates the effects of hypothermia on the monocyte inflammatory response. Surgery 2015, 158, 646–654. [Google Scholar] [CrossRef]
- Demartini, C.; Greco, R.; Zanaboni, A.M.; Sances, G.; De Icco, R.; Borsook, D.; Tassorelli, C. Nitroglycerin as a comparative experimental model of migraine pain: From animal to human and back. Prog. Neurobiol. 2019, 177, 15–32. [Google Scholar] [CrossRef]
- Villa, G.; Ceruti, S.; Zanardelli, M.; Magni, G.; Jasmin, L.; Ohara, P.T.; Abbracchio, M.P. Temporomandibular joint inflammation activates glial and immune cells in both the trigeminal ganglia and in the spinal trigeminal nucleus. Mol. Pain 2010, 6, 89. [Google Scholar] [CrossRef] [Green Version]
- Raddant, A.C.; Russo, A.F. Calcitonin gene-related peptide in migraine: Intersection of peripheral inflammation and central modulation. Expert Rev. Mol. Med. 2011, 13, e36. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Nixdorf-Bergweiler, B.E.; van Brederode, J.; Alzheimer, C.; Messlinger, K. Excitatory Effects of Calcitonin Gene-Related Peptide (CGRP) on Superficial Sp5C Neurons in Mouse Medullary Slices. Int. J. Mol. Sci. 2021, 22, 3794. [Google Scholar] [CrossRef] [PubMed]
- Messlinger, K. The big CGRP flood-sources, sinks and signalling sites in the trigeminovascular system. J. Headache Pain 2018, 19, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, R.; Demartini, C.; Zanaboni, A.M.; Tassorelli, C. Chronic and intermittent administration of systemic nitroglycerin in the rat induces an increase in the gene expression of CGRP in central areas: Potential contribution to pain processing. J. Headache Pain 2018, 19, 51. [Google Scholar] [CrossRef] [Green Version]
- Greco, R.; Demartini, C.; Francavilla, M.; Zanaboni, A.M.; Tassorelli, C. Dual Inhibition of FAAH and MAGL Counteracts Migraine-like Pain and Behavior in an Animal Model of Migraine. Cells 2021, 10, 2543. [Google Scholar] [CrossRef] [PubMed]
- Farajdokht, F.; Mohaddes, G.; Shanehbandi, D.; Karimi, P.; Babri, S. Ghrelin attenuated hyperalgesia induced by chronic nitroglycerin: CGRP and TRPV1 as targets for migraine management. Cephalalgia 2018, 38, 1716–1730. [Google Scholar] [CrossRef]
- Kunkler, P.E.; Ballard, C.J.; Oxford, G.S.; Hurley, J.H. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain 2011, 152, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Inoue, R.; Morii, T.; Takahashi, N.; Yamamoto, S.; Hara, Y.; Tominaga, M.; Shimizu, S.; Sato, Y.; Mori, Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat. Chem. Biol. 2006, 2, 596–607. [Google Scholar] [CrossRef]
- Andersson, D.A.; Gentry, C.; Moss, S.; Bevan, S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J. Neurosci. 2008, 28, 2485–2494. [Google Scholar] [CrossRef] [Green Version]
- Bautista, D.M.; Pellegrino, M.; Tsunozaki, M. TRPA1: A gatekeeper for inflammation. Annu. Rev. Physiol. 2013, 75, 181–200. [Google Scholar] [CrossRef] [Green Version]
- Wenzl, M.V.; Beretta, M.; Gorren, A.C.; Zeller, A.; Baral, P.K.; Gruber, K.; Russwurm, M.; Koesling, D.; Schmidt, K.; Mayer, B. Role of the general base Glu-268 in nitroglycerin bioactivation and superoxide formation by aldehyde dehydrogenase-2. J. Biol. Chem. 2009, 284, 19878–19886. [Google Scholar] [CrossRef] [PubMed]
- Won, L.; Kraig, R.P. Insulin-like growth factor-1 inhibits nitroglycerin-induced trigeminal activation of oxidative stress, calcitonin gene-related peptide and c-Fos expression. Neurosci. Lett. 2021, 751, 135809. [Google Scholar] [CrossRef] [PubMed]
- Reuter, U.; Chiarugi, A.; Bolay, H.; Moskowitz, M.A. Nuclear factor-kappaB as a molecular target for migraine therapy. Ann. Neurol. 2002, 51, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.; Tassorelli, C.; Cappelletti, D.; Sandrini, G.; Nappi, G. Activation of the transcription factor NF-kappaB in the nucleus trigeminalis caudalis in an animal model of migraine. Neurotoxicology 2005, 26, 795–800. [Google Scholar] [CrossRef]
- Liao, C.C.; Li, J.M.; Hsieh, C.L. Auricular Electrical Stimulation Alleviates Headache through CGRP/COX-2/TRPV1/TRPA1 Signaling Pathways in a Nitroglycerin-Induced Migraine Rat Model. Evid. Based Complement. Alternat. Med. 2019, 2019, 2413919. [Google Scholar] [CrossRef] [Green Version]
- Magni, G.; Marinelli, A.; Riccio, D.; Lecca, D.; Tonelli, C.; Abbracchio, M.P.; Petroni, K.; Ceruti, S. Purple Corn Extract as Anti-allodynic Treatment for Trigeminal Pain: Role of Microglia. Front. Cell Neurosci. 2018, 12, 378. [Google Scholar] [CrossRef]
- He, W.; Long, T.; Pan, Q.; Zhang, S.; Zhang, Y.; Zhang, D.; Qin, G.; Chen, L.; Zhou, J. Microglial NLRP3 inflammasome activation mediates IL-1β release and contributes to central sensitization in a recurrent nitroglycerin-induced migraine model. J. Neuroinflammation 2019, 16, 78. [Google Scholar] [CrossRef] [Green Version]
- Jing, F.; Zou, Q.; Wang, Y.; Cai, Z.; Tang, Y. Activation of microglial GLP-1R in the trigeminal nucleus caudalis suppresses central sensitization of chronic migraine after recurrent nitroglycerin stimulation. J. Headache Pain 2021, 22, 86. [Google Scholar] [CrossRef]
- Wen, Q.; Wang, Y.; Pan, Q.; Tian, R.; Zhang, D.; Qin, G.; Zhou, J.; Chen, L. MicroRNA-155-5p promotes neuroinflammation and central sensitization via inhibiting SIRT1 in a nitroglycerin-induced chronic migraine mouse model. J. Neuroinflammation 2021, 18, 287. [Google Scholar] [CrossRef]
- Chen, H.; Tang, X.; Li, J.; Hu, B.; Yang, W.; Zhan, M.; Ma, T.; Xu, S. IL-17 crosses the blood-brain barrier to trigger neuroinflammation: A novel mechanism in nitroglycerin-induced chronic migraine. J. Headache Pain 2022, 23, 1. [Google Scholar] [CrossRef]
- Pan, Q.; Wang, Y.; Tian, R.; Wen, Q.; Qin, G.; Zhang, D.; Chen, L.; Zhang, Y.; Zhou, J. Sphingosine-1 phosphate receptor 1 contributes to central sensitization in recurrent nitroglycerin-induced chronic migraine model. J. Headache Pain 2022, 23, 25. [Google Scholar] [CrossRef] [PubMed]
- Sago, T.; Ono, K.; Harano, N.; Furuta-Hidaka, K.; Hitomi, S.; Nunomaki, M.; Yoshida, M.; Shiiba, S.; Nakanishi, O.; Matsuo, K.; et al. Distinct time courses of microglial and astrocytic hyperactivation and the glial contribution to pain hypersensitivity in a facial cancer model. Brain Res. 2012, 1457, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Gwak, Y.S.; Kang, J.; Unabia, G.C.; Hulsebosch, C.E. Spatial and temporal activation of spinal glial cells: Role of gliopathy in central neuropathic pain following spinal cord injury in rats. Exp. Neurol. 2012, 234, 362–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mika, J.; Zychowska, M.; Popiolek-Barczyk, K.; Rojewska, E.; Przewlocka, B. Importance of glial activation in neuropathic pain. Eur. J. Pharmacol. 2013, 716, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.U.; Ying, Y.; Li, Y.; Eyo, U.B.; Chen, T.; Zheng, J.; Umpierre, A.D.; Zhu, J.; Bosco, D.B.; Dong, H.; et al. Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nat. Neurosci. 2019, 22, 1771–1781. [Google Scholar] [CrossRef]
- Cunningham, C.; Dunne, A.; Lopez-Rodriguez, A.B. Astrocytes: Heterogeneous and Dynamic Phenotypes in Neurodegeneration and Innate Immunity. Neuroscientist 2019, 25, 455–474. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Yang, Y.; Yi, J.; Zhao, Z.; Ye, R. Ablation of transient receptor potential vanilloid subtype 1-expressing neurons in rat trigeminal ganglia aggravated bone resorption in periodontitis with diabetes. Arch. Oral Biol. 2022, 133, 105293. [Google Scholar] [CrossRef]
- Franceschini, A.; Vilotti, S.; Ferrari, M.D.; van den Maagdenberg, A.M.; Nistri, A.; Fabbretti, E. TNFα levels and macrophages expression reflect an inflammatory potential of trigeminal ganglia in a mouse model of familial hemiplegic migraine. PLoS ONE 2013, 8, e52394. [Google Scholar] [CrossRef]
- Aral, L.A.; ErgÜn, M.A.; Bolay, H. Cellular iron storage and trafficking are affected by GTN stimulation in primary glial and meningeal cell culture. Turk. J. Biol. 2021, 45, 46–55. [Google Scholar] [CrossRef]
- Borkum, J.M. Brain Energy Deficit as a Source of Oxidative Stress in Migraine: A Molecular Basis for Migraine Susceptibility. Neurochem. Res. 2021, 46, 1913–1932. [Google Scholar] [CrossRef]
- Lee, S.M.; Cho, Y.S.; Kim, T.H.; Jin, M.U.; Ahn, D.K.; Noguchi, K.; Bae, Y.C. An ultrastructural evidence for the expression of transient receptor potential ankyrin 1 (TRPA1) in astrocytes in the rat trigeminal caudal nucleus. J. Chem. Neuroanat. 2012, 45, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Vellani, V.; Gomis-Perez, C.; Pinti, M.; Prandini, M.; Pavesi, G.; Giacomoni, C.; Caprini, M. TRPA1 Is Expressed in Central But Not in Peripheral Glia. J. Biomed. Sci. Eng. 2016, 9, 515–531. [Google Scholar] [CrossRef] [Green Version]
- Bosson, A.; Paumier, A.; Boisseau, S.; Jacquier-Sarlin, M.; Buisson, A.; Albrieux, M. TRPA1 channels promote astrocytic Ca2+ hyperactivity and synaptic dysfunction mediated by oligomeric forms of amyloid-β peptide. Mol. Neurodegener. 2017, 12, 53. [Google Scholar] [CrossRef] [Green Version]
- Di Castro, M.A.; Chuquet, J.; Liaudet, N.; Bhaukaurally, K.; Santello, M.; Bouvier, D.; Tiret, P.; Volterra, A. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat. Neurosci. 2011, 14, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Viana, F. TRPA1 channels: Molecular sentinels of cellular stress and tissue damage. J. Physiol. 2016, 594, 4151–4169. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.M.; Jin, K.S.; Chung, C.S. Differential effects of corticosteroids on the expression of cyclooxygenase-2, tumour necrosis factor-alpha and matrix metalloproteinase-9 in an animal model of migraine. Cephalalgia 2008, 28, 1179–1187. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kishishita, Y.; Yoshida, M.; Miura, D.; Suzuki, H.; Ishikawa, K.; Miyazaki, H.; Nojima, J.; Yamamoto, M.; Ishikawa, T. Activation of different signals identified with glia cells contribute to the progression of hyperalgesia. Cell Mol. Neurobiol. 2013, 33, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Zhang, Y.Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.Y.; Sessle, B.J.; Dostrovsky, J.O. Role of astrocytes in pain. Neurochem. Res. 2012, 37, 2419–2431. [Google Scholar] [CrossRef]
- Matejuk, A.; Ransohoff, R.M. Crosstalk Between Astrocytes and Microglia: An Overview. Front. Immunol 2020, 11, 1416. [Google Scholar] [CrossRef]
- Qi, J.; Chen, C.; Meng, Q.X.; Wu, Y.; Wu, H.; Zhao, T.B. Crosstalk between Activated Microglia and Neurons in the Spinal Dorsal Horn Contributes to Stress-induced Hyperalgesia. Sci. Rep. 2016, 6, 39442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Wang, W.; Mei, X.; Huang, J.; Wei, Y.; Wang, Y.; Wu, S.; Li, Y. Crosstalk between spinal astrocytes and neurons in nerve injury-induced neuropathic pain. PLoS ONE 2009, 4, e6973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helley, M.P.; Abate, W.; Jackson, S.K.; Bennett, J.H.; Thompson, S.W. The expression of Toll-like receptor 4, 7 and co-receptors in neurochemical sub-populations of rat trigeminal ganglion sensory neurons. Neuroscience 2015, 310, 686–698. [Google Scholar] [CrossRef] [Green Version]
- Park, C.K.; Xu, Z.Z.; Berta, T.; Han, Q.; Chen, G.; Liu, X.J.; Ji, R.R. Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron 2014, 82, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Gao, Y.J.; Ji, R.R. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci. Bull. 2012, 28, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Dubin, A.E.; Petrus, M.J.; Patapoutian, A. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS ONE 2009, 4, e7596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thalakoti, S.; Patil, V.V.; Damodaram, S.; Vause, C.V.; Langford, L.E.; Freeman, S.E.; Durham, P.L. Neuron-glia signaling in trigeminal ganglion: Implications for migraine pathology. Headache 2007, 47, 1008–1023. [Google Scholar] [CrossRef] [Green Version]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef] [Green Version]
- Clavelou, P.; Dallel, R.; Orliaguet, T.; Woda, A.; Raboisson, P. The orofacial formalin test in rats: Effects of different formalin concentrations. Pain 1995, 62, 295–301. [Google Scholar] [CrossRef]
- Caminski, E.S.; de Freitas, L.M.; Dallegrave, E.; Junior, C.; Gomez, M.V.; Pereira, E.; Antunes, F.; de Souza, A.H. Analgesic effects of the CTK 01512-2 toxin in different models of orofacial pain in rats. Pharmacol. Rep. 2020, 72, 600–611. [Google Scholar] [CrossRef]
- Fischer, M.J.; Soller, K.J.; Sauer, S.K.; Kalucka, J.; Veglia, G.; Reeh, P.W. Formalin evokes calcium transients from the endoplasmatic reticulum. PLoS ONE 2015, 10, e0123762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gualdani, R.; Ceruti, S.; Magni, G.; Merli, D.; Di Cesare Mannelli, L.; Francesconi, O.; Richichi, B.; la Marca, G.; Ghelardini, C.; Moncelli, M.R.; et al. Lipoic-based TRPA1/TRPV1 antagonist to treat orofacial pain. ACS Chem. Neurosci. 2015, 6, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Vosough, E.; Rahimi, V.B.; Masoud, S.A.; Mirkarimi, H.R.; Demneh, M.K.; Abed, A.; Banafshe, H.R.; Askari, V.R. Evaluation of protective effects of non-selective cannabinoid receptor agonist WIN 55,212-2 against the nitroglyc-erine-induced acute and chronic animal models of migraine: A mechanistic study. Life Sci. 2019, 232, 116670. [Google Scholar] [CrossRef] [PubMed]
- Moye, L.S.; Tipton, A.F.; Dripps, I.; Sheets, Z.; Crombie, A.; Violin, J.D.; Pradhan, A.A. Delta opioid receptor agonists are effective for multiple types of headache disorders. Neuropharmacology 2019, 148, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Demartini, C.; Greco, R.; Zanaboni, A.M.; Francesconi, O.; Nativi, C.; Tassorelli, C.; Deseure, K. Antagonism of Transient Receptor Potential Ankyrin Type-1 Channels as a Potential Target for the Treatment of Trigeminal Neuropathic Pain: Study in an Animal Model. Int. J. Mol. Sci. 2018, 19, 3320. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, E.S.; La, J.H.; Scheff, N.N.; Davis, B.M.; Albers, K.M.; Gebhart, G.F. TRPV1 and TRPA1 antagonists prevent the transition of acute to chronic inflammation and pain in chronic pancreatitis. J. Neurosci. 2013, 33, 5603–5611. [Google Scholar] [CrossRef] [Green Version]
- Koivisto, A.; Hukkanen, M.; Saarnilehto, M.; Chapman, H.; Kuokkanen, K.; Wei, H.; Viisanen, H.; Akerman, K.E.; Lindstedt, K.; Pertovaara, A. Inhibiting TRPA1 ion channel reduces loss of cutaneous nerve fiber function in diabetic animals: Sustained activation of the TRPA1 channel contributes to the pathogenesis of peripheral diabetic neuropathy. Pharmacol. Res. 2012, 65, 149–158. [Google Scholar] [CrossRef]
- Koivisto, A.; Jalava, N.; Bratty, R.; Pertovaara, A. TRPA1 Antagonists for Pain Relief. Pharmaceuticals 2018, 11, 117. [Google Scholar] [CrossRef] [Green Version]
- Greco, R.; Demartini, C.; Zanaboni, A.M.; Tumelero, E.; Reggiani, A.; Misto, A.; Piomelli, D.; Tassorelli, C. FAAH inhibition as a preventive treatment for migraine: A pre-clinical study. Neurobiol. Dis. 2020, 134, 104624. [Google Scholar] [CrossRef]
- Greco, R.; Bandiera, T.; Mangione, A.S.; Demartini, C.; Siani, F.; Nappi, G.; Sandrini, G.; Guijarro, A.; Armirotti, A.; Piomelli, D.; et al. Effects of peripheral FAAH blockade on NTG-induced hyperalgesia--evaluation of URB937 in an animal model of migraine. Cephalalgia 2015, 35, 1065–1076. [Google Scholar] [CrossRef] [Green Version]
- Greco, R.; Demartini, C.; Zanaboni, A.M.; Redavide, E.; Pampalone, S.; Toldi, J.; Fülöp, F.; Blandini, F.; Nappi, G.; Sandrini, G.; et al. Effects of kynurenic acid analogue 1 (KYNA-A1) in nitroglycerin-induced hyperalgesia: Targets and anti-migraine mechanisms. Cephalalgia 2017, 37, 1272–1284. [Google Scholar] [CrossRef] [PubMed]
- Colburn, R.W.; DeLeo, J.A.; Rickman, A.J.; Yeager, M.P.; Kwon, P.; Hickey, W.F. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J. Neuroimmunol. 1997, 79, 163–175. [Google Scholar] [CrossRef]
- Peruzzaro, S.T.; Andrews, M.; Al-Gharaibeh, A.; Pupiec, O.; Resk, M.; Story, D.; Maiti, P.; Rossignol, J.; Dunbar, G.L. Transplantation of mesenchymal stem cells genetically engineered to overexpress interleukin-10 promotes alternative inflammatory response in rat model of traumatic brain injury. J. Neuroinflammation 2019, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.; Demartini, C.; Zanaboni, A.; Casini, I.; De Icco, R.; Reggiani, A.; Misto, A.; Piomelli, D.; Tassorelli, C. Characterization of the peripheral FAAH inhibitor, URB937, in animal models of acute and chronic migraine. Neurobiol. Dis. 2021, 147, 105157. [Google Scholar] [CrossRef]








| Group Name | NTG/Control Treatment (i.p. Injection) | ADM_12/Control Treatment (i.p. Injection) | OFT (N) | Real Time- PCR (N) | IF (N) | |
|---|---|---|---|---|---|---|
| ACUTE MODEL | CT | acute NTG vehicle | saline | 11 | 6 | 5 |
| NTG | acute NTG (10 mg/kg) | saline | 11 | 6 | 5 | |
| ADM_12 | acute NTG vehicle | ADM_12 (30 mg/kg) | 12 | 6 | 6 | |
| NTG + ADM_12 | acute NTG (10 mg/kg) | ADM_12 (30 mg/kg) | 12 | 6 | 6 | |
| CHRONIC MODEL | CT chronic | chronic NTG vehicle | saline | 12 | 6 | 6 |
| NTG chronic | chronic NTG (5 mg/kg) | saline | 13 | 6 | 7 | |
| ADM_12 chronic | chronic NTG vehicle | ADM_12 (30 mg/kg) | 12 | 6 | 6 | |
| NTG + ADM_12 chronic | chronic NTG (5 mg/kg) | ADM_12 (30 mg/kg) | 11 | 6 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demartini, C.; Greco, R.; Magni, G.; Zanaboni, A.M.; Riboldi, B.; Francavilla, M.; Nativi, C.; Ceruti, S.; Tassorelli, C. Modulation of Glia Activation by TRPA1 Antagonism in Preclinical Models of Migraine. Int. J. Mol. Sci. 2022, 23, 14085. https://doi.org/10.3390/ijms232214085
Demartini C, Greco R, Magni G, Zanaboni AM, Riboldi B, Francavilla M, Nativi C, Ceruti S, Tassorelli C. Modulation of Glia Activation by TRPA1 Antagonism in Preclinical Models of Migraine. International Journal of Molecular Sciences. 2022; 23(22):14085. https://doi.org/10.3390/ijms232214085
Chicago/Turabian StyleDemartini, Chiara, Rosaria Greco, Giulia Magni, Anna Maria Zanaboni, Benedetta Riboldi, Miriam Francavilla, Cristina Nativi, Stefania Ceruti, and Cristina Tassorelli. 2022. "Modulation of Glia Activation by TRPA1 Antagonism in Preclinical Models of Migraine" International Journal of Molecular Sciences 23, no. 22: 14085. https://doi.org/10.3390/ijms232214085