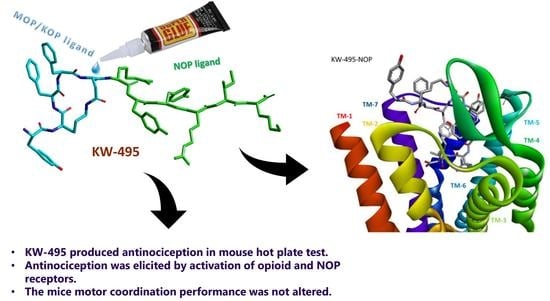
Synthesis, Biological Activity and Molecular Docking of Chimeric Peptides Targeting Opioid and NOP Receptors
Abstract
:1. Introduction
2. Results
2.1. Design of Opioid/NOP Chimeras
2.2. Pharmacological Characterization of the Chimeric Peptides In Vitro
2.3. Antinociceptive Activity
2.4. Receptor Antagonist Experiments
2.5. Motor Performance Study (Rotarod Test)
2.6. Computational Studies

3. Discussion
4. Materials and Methods
4.1. General Methods
4.2. Peptide Synthesis
4.3. Cell Culture
4.4. Membrane Preparation
4.5. [3H]DAMGO, [3H]deltorphin-2, [3H]U-69593 Displacement Binding Assay
4.6. [Leucyl-3H]nociceptin Displacement Binding Assay
4.7. Binding Data Analysis
4.8. Calcium Mobilization Functional Assay
4.9. Functional Assay Data Analysis and Terminology
4.10. In Vivo Studies
4.10.1. Animals
4.10.2. Drugs and Pharmacological Treatment
4.10.3. Assessment of Antinociception
4.10.4. Assessment of Motor Coordination
4.11. Molecular Docking
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janecka, A.; Fichna, J.; Janecki, T. Opioid receptors and their ligands. Curr. Top. Med. Chem. 2004, 4, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Günther, T.; Dasgupta, P.; Mann, A.; Miess, E.; Kliewer, A.; Fritzwanker, S.; Steinborn, R.; Schulz, S. Targeting multiple opioid receptors—Improved analgesics with reduced side effects? Br. J. Pharmacol. 2018, 175, 2857–2868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuckit, M.A. Treatment of Opioid-Use Disorders. N. Engl. J. Med. 2016, 375, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Darcq, E.; Kieffer, B.L. Opioid receptors: Drivers to addiction? Nat. Rev. Neurosci. 2018, 19, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Li, Q.; Dong, Y.; Yan, W.; Song, K.; Lin, Y.Q.; Sun, Y.G. Mu-Opioid Receptors Expressed in Glutamatergic Neurons are Essential for Morphine Withdrawal. Neurosci. Bull. 2020, 36, 1095–1106. [Google Scholar] [CrossRef]
- Mollereau, C.; Parmentier, M.; Mailleux, P.; Butour, J.L.; Moisand, C.; Chalon, P.; Caput, D.; Vassart, G.; Meunier, J.C. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994, 341, 33–38. [Google Scholar] [CrossRef]
- Meunier, J.C.; Mollereau, C.; Toll, L.; Suaudeau, C.; Moisand, C.; Alvinerie, P.; Butour, J.L.; Guillemot, J.C.; Ferrara, P.; Monsarrat, B.; et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 1995, 377, 532–535. [Google Scholar] [CrossRef]
- Reinscheid, R.K.; Nothacker, H.P.; Bourson, A.; Ardati, A.; Henningsen, R.A.; Bunzow, J.R.; Grandy, D.K.; Langen, H.; Monsma, F.J., Jr.; Civelli, O. Orphanin FQ: A neuropeptide that activates an opioidlike G protein-coupled receptor. Science 1995, 270, 792–794. [Google Scholar] [CrossRef] [PubMed]
- Zaveri, N.T. Nociceptin Opioid Receptor (NOP) as a Therapeutic Target: Progress in Translation from Preclinical Research to Clinical Utility. J. Med. Chem. 2016, 59, 7011–7028. [Google Scholar] [CrossRef] [Green Version]
- Mustazza, C.; Bastanzio, G. Development of nociceptin receptor (NOP) agonists and antagonists. Med. Res. Rev. 2011, 31, 605–648. [Google Scholar] [CrossRef] [PubMed]
- Mika, J.; Obara, I.; Przewlocka, B. The role of nociceptin and dynorphin in chronic pain: Implications of neuro-glial interaction. Neuropeptides 2011, 45, 247–261. [Google Scholar] [CrossRef]
- Corradini, L.; Briscini, L.; Ongini, E.; Bertorelli, R. The putative OP(4) antagonist, [Nphe(1)]nociceptin(1-13)NH(2), prevents the effects of nociceptin in neuropathic rats. Brain Res. 2001, 905, 127–133. [Google Scholar] [CrossRef]
- Lambert, D.G. The nociceptin/orphanin FQ receptor: A target with broad therapeutic potential. Nat. Rev. Drug Discov. 2008, 7, 694–710. [Google Scholar] [CrossRef] [PubMed]
- Khroyan, T.V.; Polgar, W.E.; Orduna, J.; Montenegro, J.; Jiang, F.; Zaveri, N.T.; Toll, L. Differential effects of nociceptin/orphanin FQ (NOP) receptor agonists in acute versus chronic pain: Studies with bifunctional NOP/μ receptor agonists in the sciatic nerve ligation chronic pain model in mice. J. Pharmacol. Exp. Ther. 2011, 339, 687–693. [Google Scholar] [CrossRef] [Green Version]
- Micheli, L.; Lucarini, E.; Corti, F.; Ciccocioppo, R.; Calò, G.; Rizzi, A.; Ghelardini, C.; Di Cesare Mannelli, L. Involvement of the N/OFQ-NOP system in rat morphine antinociceptive tolerance: Are astrocytes the crossroad? Eur. J. Pharmacol. 2018, 823, 79–86. [Google Scholar] [CrossRef]
- Calo, G.; Lambert, D. Nociceptin/orphanin FQ receptor ligands and translational challenges: Focus on cebranopadol as an innovative analgesic. Br. J. Anaesth. 2018, 121, 1105–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toll, L.; Bruchas, M.R.; Cox, B.M.; Zaveri, N.T. Nociceptin/orphanin FQ receptor structure, signaling, ligands, functions, and interactions with opioid systems. Pharmacol. Rev. 2016, 68, 419–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granier, S.; Manglik, A.; Kruse, A.C.; Kobilka, T.S.; Thian, F.S.; Weis, W.I.; Kobilka, B.K. Structure of the δ-opioid receptor bound to naltrindole. Nature 2012, 485, 400–404. [Google Scholar] [CrossRef] [Green Version]
- Manglik, A.; Kruse, A.C.; Kobilka, T.S.; Thian, F.S.; Mathiesen, J.M.; Sunahara, R.K.; Pardo, L.; Weis, W.I.; Kobilka, B.K.; Granier, S. Crystal structure of the µ-opioid receptor bound to a morphinan antagonist. Nature 2012, 485, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Thompson, A.A.; Liu, W.; Chun, E.; Katritch, V.; Wu, H.; Vardy, E.; Huang, X.P.; Trapella, C.; Guerrini, R.; Calo, G.; et al. Structure of the nociceptin/orphanin FQ receptor in complex with a peptide mimetic. Nature 2012, 485, 395–399. [Google Scholar] [CrossRef]
- Wu, H.; Wacker, D.; Mileni, M.; Katritch, V.; Han, G.W.; Vardy, E.; Liu, W.; Thompson, A.A.; Huang, X.P.; Carroll, F.I.; et al. Structure of the human κ-opioid receptor in complex with JDTic. Nature 2012, 485, 327–332. [Google Scholar] [CrossRef]
- Caló, G.; Guerrini, R. Medicinal chemistry, pharmacology, and biological actions of peptide ligands selective for the nociceptin/orphanin FQ receptor. In Research and Development of Opioid-Related Ligands; Mei-Chuan Ko, M.-C., Husbands, S.M., Eds.; ACS Publications: Washington, DC, USA, 2013; Volume 1131, pp. 275–325. [Google Scholar]
- Schröder, W.; Lambert, D.G.; Ko, M.C.; Koch, T. Functional plasticity of the N/OFQ-NOP receptor system determines analgesic properties of NOP receptor agonists. Br. J. Pharmacol. 2014, 171, 3777–3800. [Google Scholar] [CrossRef] [Green Version]
- Morphy, R.; Rankovic, Z. Designing multiple ligands—Medicinal chemistry strategies and challenges. Curr. Pharm. Des. 2009, 15, 587–600. [Google Scholar] [CrossRef]
- Dietis, N.; Guerrini, R.; Calo, G.; Salvadori, S.; Rowbotham, D.J.; Lambert, D.G. Simultaneous targeting of multiple opioid receptors: A strategy to improve side-effect profile. Br. J. Anaesth. 2009, 103, 38–49. [Google Scholar] [CrossRef] [Green Version]
- Dvoracsko, S.; Stefanucci, A.; Novellino, E.; Mollica, A. The design of multitarget ligands for chronic and neuropathic pain. Future Med. Chem. 2015, 7, 2469–2483. [Google Scholar] [CrossRef]
- Mansour, A.; Fox, C.A.; Akil, H.; Watson, S.J. Opioid-receptor mRNA expression in the rat CNS: Anatomical and functional implications. Trends Neurosci. 1995, 18, 22–29. [Google Scholar] [CrossRef]
- Neal, C.R., Jr.; Mansour, A.; Reinscheid, R.; Nothacker, H.P.; Civelli, O.; Akil, H.; Watson, S.J., Jr. Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: Comparison of ORL1 receptor mRNA expression with 125I-[14Tyr]-orphanin FQ binding. J. Comp. Neurol. 1999, 412, 563–605. [Google Scholar] [CrossRef]
- Cunningham, C.W.; Elballa, W.M.; Vold, S.U. Bifunctional opioid receptor ligands as novel analgesics. Neuropharmacology 2019, 151, 195–207. [Google Scholar] [CrossRef]
- Kiguchi, N.; Ding, H.; Ko, M.C. Therapeutic potentials of NOP and MOP receptor coactivation for the treatment of pain and opioid abuse. J. Neurosci. Res. 2020, 100, 191–202. [Google Scholar] [CrossRef]
- Guillemyn, K.; Starnowska, J.; Lagard, C.; Dyniewicz, J.; Rojewska, E.; Mika, J.; Chung, N.N.; Utard, V.; Kosson, P.; Lipkowski, A.W.; et al. Bifunctional peptide-based opioid agonist-nociceptin antagonist ligands for dual treatment of acute and neuropathic pain. J. Med. Chem. 2016, 59, 3777–3792. [Google Scholar] [CrossRef]
- Lagard, C.; Chevillard, L.; Guillemyn, K.; Risède, P.; Laplanche, J.-L.; Spetea, M.; Ballet, S.; Mégarbane, B. Bifunctional peptide-based opioid agonist/nociceptin antagonist ligand for dual treatment of nociceptive and neuropathic pain. Pain 2017, 158, 505. [Google Scholar] [CrossRef] [Green Version]
- Evans, R.M.; You, H.; Hameed, S.; Altier, C.; Mezghrani, A.; Bourinet, E.; Zamponi, G.W. Heterodimerization of ORL1 and opioid receptors and its consequences for N-type calcium channel regulation. J. Biol. Chem. 2010, 285, 1032–1040. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.-X.; Bolan, E.; Pasternak, G.W. Dimerization of morphine and orphanin FQ/nociceptin receptors: Generation of a novel opioid receptor subtype. Biochem. Biophys. Res. Commun. 2002, 297, 659–663. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.-T.; Hang, L.; Liu, T. Mu Opioid Receptor Heterodimers Emerge as Novel Therapeutic Targets: Recent Progress and Future. Front. Pharmacol. 2020, 11, 1078. [Google Scholar] [CrossRef]
- Linz, K.; Christoph, T.; Tzschentke, T.M.; Koch, T.; Schiene, K.; Gautrois, M.; Schröder, W.; Kögel, B.Y.; Beier, H.; Englberger, W.; et al. Cebranopadol: A novel potent analgesic nociceptin/orphanin FQ peptide and opioid receptor agonist. J. Pharmacol. Exp. Ther. 2014, 349, 535–548. [Google Scholar] [CrossRef] [Green Version]
- Rizzi, A.; Cerlesi, M.C.; Ruzza, C.; Malfacini, D.; Ferrari, F.; Bianco, S.; Costa, T.; Guerrini, R.; Trapella, C.; Caló, G. Pharmacological characterization of cebranopadol a novel analgesic acting as mixed nociceptin/orphanin FQ and opioid receptor agonist. Pharmacol. Res. Perspect. 2016, 4, e00247. [Google Scholar] [CrossRef]
- Khroyan, T.V.; Zaveri, N.T.; Polgar, W.E.; Orduna, J.; Olsen, C.; Jiang, F.; Toll, L. [1-(1-(bicyclo [3.3.1] nonan-9-yl) piperidin-4-yl) indolin-2-one], a novel mixed nociceptin/orphanin FQ/μ-opioid receptor partial agonist: Analgesic and rewarding properties in mice. J. Pharmacol. Exp. Ther. 2007, 320, 934–943. [Google Scholar] [CrossRef]
- Sukhtankar, D.D.; Zaveri, N.T.; Husbands, S.M.; Ko, M.-C. Effects of spinally administered bifunctional nociceptin/orphanin FQ peptide receptor/μ-opioid receptor ligands in mouse models of neuropathic and inflammatory pain. J. Pharmacol. Exp. Ther. 2013, 346, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; Kiguchi, N.; Yasuda, D.; Daga, P.R.; Polgar, W.E.; Lu, J.J.; Czoty, P.W.; Kishioka, S.; Zaveri, N.T.; Ko, M.-C. A bifunctional nociceptin and mu opioid receptor agonist is analgesic without opioid side effects in nonhuman primates. Sci. Transl. Med. 2018, 10, eaar3483. [Google Scholar] [CrossRef] [Green Version]
- Dooley, C.T.; Spaeth, C.G.; Berzetei-Gurske, I.P.; Craymer, K.; Adapa, I.D.; Brandt, S.R.; Houghten, R.A.; Toll, L. Binding and in vitro activities of peptides with high affinity for the nociceptin/orphanin FQ receptor, ORL1. J. Pharmacol. Exp. Ther. 1997, 283, 735–741. [Google Scholar]
- Berger, H.; Bigoni, R.; Albrecht, E.; Richter, R.M.; Krause, E.; Bienert, M.; Caló, G. The nociceptin/orphanin FQ receptor ligand acetyl-RYYRIK-amide exhibits antagonistic and agonistic properties. Peptides 2000, 21, 1131–1139. [Google Scholar] [CrossRef]
- Calo, G.; Bigoni, R.; Rizzi, A.; Guerrini, R.; Salvadori, S.; Regoli, D. Nociceptin/orphanin FQ receptor ligands. Peptides 2000, 21, 935–947. [Google Scholar] [CrossRef]
- Perlikowska, R.; do-Rego, J.C.; Cravezic, A.; Fichna, J.; Wyrebska, A.; Toth, G.; Janecka, A. Synthesis and biological evaluation of cyclic endomorphin-2 analogs. Peptides 2010, 31, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Camarda, V.; Fischetti, C.; Anzellotti, N.; Molinari, P.; Ambrosio, C.; Kostenis, E.; Regoli, D.; Trapella, C.; Guerrini, R.; Severo, S.; et al. Pharmacological profile of NOP receptors coupled with calcium signaling via the chimeric protein G alpha qi5. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 379, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Camarda, V.; Caló, G. Chimeric G proteins in fluorimetric calcium assays: Experience with opioid receptors. Methods Mol. Biol. 2013, 937, 293–306. [Google Scholar]
- Piekielna, J.; Gentilucci, L.; De Marco, R.; Perlikowska, R.; Adamska, A.; Olczak, J.; Mazur, M.; Artali, R.; Modranka, J.; Janecki, T.; et al. Cyclic side-chain-linked opioid analogs utilizing cis- and trans-4-aminocyclohexyl-D-alanine. Bioorg. Med. Chem. 2014, 22, 6545–6551. [Google Scholar]
- Ma, H.; Li, M.; Pagare, P.P.; Wang, H.; Nassehi, N.; Santos, E.J.; Negus, S.S.; Selley, D.E.; Zhang, Y. Novel bivalent ligands carrying potential antinociceptive effects by targeting putative mu opioid receptor and chemokine receptor CXCR4 heterodimers. Bioorg. Chem. 2022, 120, 105641. [Google Scholar] [CrossRef]
- Yuan, Y.; Arnatt, C.K.; El-Hage, N.; Dever, S.M.; Jacob, J.C.; Selley, D.E.; Hauserb, K.F.; Zhang, Y. A bivalent ligand targeting the putative mu opioid receptor and chemokine receptor CCR5 heterodimer: Binding affinity versus functional activities. Med. Chem. Commun. 2013, 4, 847–851. [Google Scholar] [CrossRef] [Green Version]
- Huey, R.; Morris, G.M.; Olson, A.J.; Goodsell, D.S. A semiempirical free energy force field with charge-based desolvation. J. Comput. Chem. 2007, 28, 1145–1152. [Google Scholar] [CrossRef]
- Che, T.; Majumdar, S.; Zaidi, S.A.; Ondachi, P.; McCorvy, J.D.; Wang, S.; Mosier, P.D.; Uprety, R.; Vardy, E.; Krumm, B.E.; et al. Structure of the Nanobody-Stabilized Active State of the Kappa Opioid Receptor. Cell 2018, 172, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Piekielna-Ciesielska, J.; Artali, R.; Azzam, A.A.H.; Lambert, D.G.; Kluczyk, A.; Gentilucci, L.; Janecka, A. Pharmacological Characterization of µ-Opioid Receptor Agonists with Biased G Protein or β-Arrestin Signaling, and Computational Study of Conformational Changes during Receptor Activation. Molecules 2020, 26, 13. [Google Scholar] [CrossRef]
- Gentilucci, L.; Tolomelli, A.; De Marco, R.; Artali, R. Molecular Docking of Opiates and Opioid Peptides, a Tool for the Design of Selective Agonists and Antagonists, and for the Investigation of Atypical Ligand-Receptor Interactions. Curr. Med. Chem. 2012, 19, 1587–1601. [Google Scholar] [CrossRef] [PubMed]
- De Marco, R.; Bedini, A.; Spampinato, S.; Comellini, L.; Zhao, J.; Artali, R.; Gentilucci, L. Constraining Endomorphin-1 by β,α-Hybrid Dipeptide/Heterocycle Scaffolds: Identification of a Novel κ-Opioid Receptor Selective Partial Agonist. J. Med. Chem. 2018, 61, 5751–5757. [Google Scholar] [CrossRef]
- O’Connor, C.; White, K.L.; Doncescu, N.; Didenko, T.; Roth, B.L.; Czaplicki, G.; Stevens, R.C.; Wüthrich, K.; Milon, A. NMR structure and dynamics of the agonist dynorphin peptide bound to the human kappa opioid receptor. Proc. Natl. Acad. Sci. USA 2015, 112, 11852–11857. [Google Scholar] [CrossRef] [Green Version]
- PacDOCK. Available online: https://pegasus.lbic.unibo.it/pacdock/ (accessed on 12 October 2022).
- Carbone, J.; Ghidini, A.; Romano, A.; Gentilucci, L.; Musiani, F. PacDOCK: A Web Server for Positional Distance-Based and Interaction-Based Analysis of Docking Results. Molecules 2022, 27, 6884. [Google Scholar] [CrossRef]
- Kawano, S.; Ito, R.; Nishiyama, M.; Kubo, M.; Matsushima, T.; Minamisawa, M.; Ambo, A.; Sasaki, Y. Receptor binding properties and antinociceptive effects of chimeric peptides consisting of a μ-opioid receptor agonist and an ORL1 receptor antagonist. Biol. Pharm. Bull. 2007, 30, 1260–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdei, A.I.; Borbély, A.; Magyar, A.; Szűcs, E.; Ötvös, F.; Gombos, D.; Al-Khrasani, M.; Stefanucci, A.; Dimmito, M.P.; Luisi, G.; et al. Biochemical and pharmacological investigation of novel nociceptin/OFQ analogues and N/OFQ-RYYRIK hybrid peptides. Peptides 2019, 112, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, R.L.; Thompson, A.A.; Trapella, C.; Guerrini, R.; Malfacini, D.; Patel, N.; Han, G.W.; Cherezov, V.; Caló, G.; Katritch, V.; et al. The Importance of Ligand-Receptor Conformational Pairs in Stabilization: Spotlight on the N/OFQ G Protein-Coupled Receptor. Structure 2015, 23, 2291–2299. [Google Scholar] [CrossRef] [Green Version]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef]
- Mogil, J.S.; Grisel, J.E.; Zhangs, G.; Belknap, J.K.; Grandy, D.K. Functional antagonism of mu-, delta- and kappa-opioid antinociception by orphanin FQ. Neurosci. Lett. 1996, 214, 131–134. [Google Scholar] [CrossRef]
- Tian, J.H.; Xu, W.; Fang, Y.; Mogil, J.S.; Grisel, J.E.; Grandy, D.K.; Han, J.S. Bidirectional modulatory effect of orphanin FQ on morphine-induced analgesia: Antagonism in brain and potentiation in spinal cord of the rat. Br. J. Pharmacol. 1997, 120, 676–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, S.; Ambo, A.; Sasaki, Y. Synthesis and receptor binding properties of chimeric peptides containing a μ-opioid receptor ligand and nociceptin/orphanin FQ receptor ligand Ac-RYYRIK-amide. Bioorg. Med. Chem. Lett. 2006, 16, 4839–4841. [Google Scholar] [CrossRef] [PubMed]
- Starnowska, J.; Guillemyn, K.; Makuch, W.; Mika, J.; Ballet, S.; Przewlocka, B. Bifunctional opioid/nociceptin hybrid KGNOP1 effectively attenuates pain-related behaviour in a rat model of neuropathy. Eur. J. Pharm. Sci. 2017, 104, 221–229. [Google Scholar] [CrossRef]
- Dumitrascuta, M.; Bermudez, M.; Trovato, O.; De Neve, J.; Ballet, S.; Wolber, G.; Spetea, M. Antinociceptive Efficacy of the µ-Opioid/Nociceptin Peptide-Based Hybrid KGNOP1 in Inflammatory Pain without Rewarding Effects in Mice: An Experimental Assessment and Molecular Docking. Molecules 2021, 26, 3267. [Google Scholar] [CrossRef] [PubMed]
- Erdei, A.I.; Borbély, A.; Magyar, A.; Taricska, N.; Perczel, A.; Zsíros, O.; Garab, G.; Szűcs, E.; Ötvös, F.; Zádor, F.; et al. Biochemical and pharmacological characterization of three opioid-nociceptin hybrid peptide ligands reveals substantially differing modes of their actions. Peptides 2018, 99, 205–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daga, P.R.; Zaveri, N.T. Homology modeling and molecular dynamics simulations of the active state of the nociceptin receptor reveal new insights into agonist binding and activation. Proteins 2012, 80, 1948–1961. [Google Scholar] [CrossRef] [Green Version]
- Fischetti, C.; Camarda, V.; Rizzi, A.; Pelà, M.; Trapella, C.; Guerrini, R.; McDonald, J.; Lambert, D.G.; Salvadori, S.; Regoli, D.; et al. Pharmacological characterization of the nociceptin/orphanin FQ receptor non peptide antagonist Compound 24. Eur. J. Pharmacol. 2009, 614, 50–57. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Cheng, Y.; Prusoff, W.H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar]
- Piekielna-Ciesielska, J.; Mollica, A.; Pieretti, S.; Fichna, J.; Szymaszkiewicz, A.; Zielińska, M.; Kordek, R.; Janecka, A. Antinociceptive potency of a fluorinated cyclopeptide Dmt-c[D-Lys-Phe-p-CF3-Phe-Asp]NH2. J. Enzyme Inhib. Med. Chem. 2018, 33, 560–566. [Google Scholar] [CrossRef] [Green Version]
- Hylden, J.L.; Wilcox, G.L. Antinociceptive effect of morphine on rat spinothalamic tract and other dorsal horn neurons. Neuroscience 1986, 19, 393–401. [Google Scholar] [CrossRef]
- Litchfield, J.T., Jr.; Wilcox, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar] [PubMed]
- Dunham, N.W.; Miya, T.S. A note on a simple apparatus for detecting neurological deficit in rats and mice. J. Am. Pharm Assoc. 1957, 46, 208–209. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Jakalian, A.; Bush, B.L.; Jack, D.B.; Bayly, C.I. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: I. Method. J. Comput. Chem. 2000, 21, 132–146. [Google Scholar] [CrossRef]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef]





| Compd. | Sequence | pKi | |||
|---|---|---|---|---|---|
| MOP | DOP | KOP | NOP | ||
| EM-2 | Tyr-Pro-Phe-Phe-NH2 | 9.14 ± 0.08 | <6 | <6 | N.D. |
| N/OFQ | Phe-Gly-Gly-Phe-Thr-Gly-Ala-Arg-Lys-Ser-Ala-Arg-Lys-Leu-Ala-Asn-Gln | N.D. | N.D. | N.D. | 9.09 ± 0.24 |
| Ac-RYYRIK-NH2 | Ac-Arg-Tyr-Tyr-Arg-Ile-Lys-NH2 | N.D. | N.D. | N.D. | 9.29 ± 0.85 |
| RP-170 | Tyr-c[D-Lys-Phe-Phe-Asp]-NH2 | 9.21 ± 0.05 | 6.53 ± 1.21 | 8.53 ± 0.09 | N.D. |
| KW-495 | Tyr-c[D-Lys-Phe-Phe-Asp]-Arg-Tyr-Tyr-Arg-Ile-Lys-NH2 | 8.35 ± 1.11 | <6 | 8.40 ± 2.12 | 8.65 ± 0.42 |
| KW-496 | Tyr-c[D-Lys-Phe-Phe-Asp]-Gly-Gly-Gly-Arg-Tyr-Tyr-Arg-Ile-Lys-NH2 | 8.33 ± 0.79 | <6 | 9.10 ± 0.87 | 7.35 ± 0.13 |
| Peptide | MOP | DOP | KOP | NOP | ||||
|---|---|---|---|---|---|---|---|---|
| pEC50 a (CL95%) | α b ± SEM | pEC50 (CL95%) | α ± SEM | pEC50 (CL95%) | α ± SEM | pEC50 (CL95%) | α ± SEM | |
| EM-2 | 8.08 ± 0.06 | 1.00 | inactive c | inactive | inactive | |||
| DPDPE | inactive | 7.23 ± 0.22 | 1.00 | inactive | inactive | |||
| dynorphin A | 6.67 ± 0.50 a | 0.83 ± 0.10 | 7.73 ± 0.27 | 1.00 | 8.78 ± 0.05 | 1.00 | inactive | |
| N/OFQ | inactive | inactive | inactive | 9.26 ± 0.45 | 1.0 | |||
| RP-170 | 8.93 ± 0.05 | 1.00 | inactive | 8.60 ± 0.14 | 1.00 ± 0.03 | inactive | ||
| Ac-RYYRIK-NH2 | inactive | inactive | inactive | 7.87 ± 0.49 | 0.80 ± 0.06 | |||
| KW-495 | 8.06 ± 0.46 | 0.83 ± 0.07 | inactive | 8.55 ± 0.17 | 1.02 ± 0.03 | 7.12 ± 0.13 | 0.69 ± 0.10 | |
| KW-496 | 8.31 ± 0.30 | 0.95 ± 0.07 | inactive | 8.94 ± 0.18 | 1.03 ± 0.02 | Crc incomplete at 1 μM, 0.33 ± 0.10 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wtorek, K.; Ghidini, A.; Gentilucci, L.; Adamska-Bartłomiejczyk, A.; Piekielna-Ciesielska, J.; Ruzza, C.; Sturaro, C.; Calò, G.; Pieretti, S.; Kluczyk, A.; et al. Synthesis, Biological Activity and Molecular Docking of Chimeric Peptides Targeting Opioid and NOP Receptors. Int. J. Mol. Sci. 2022, 23, 12700. https://doi.org/10.3390/ijms232012700
Wtorek K, Ghidini A, Gentilucci L, Adamska-Bartłomiejczyk A, Piekielna-Ciesielska J, Ruzza C, Sturaro C, Calò G, Pieretti S, Kluczyk A, et al. Synthesis, Biological Activity and Molecular Docking of Chimeric Peptides Targeting Opioid and NOP Receptors. International Journal of Molecular Sciences. 2022; 23(20):12700. https://doi.org/10.3390/ijms232012700
Chicago/Turabian StyleWtorek, Karol, Alessia Ghidini, Luca Gentilucci, Anna Adamska-Bartłomiejczyk, Justyna Piekielna-Ciesielska, Chiara Ruzza, Chiara Sturaro, Girolamo Calò, Stefano Pieretti, Alicja Kluczyk, and et al. 2022. "Synthesis, Biological Activity and Molecular Docking of Chimeric Peptides Targeting Opioid and NOP Receptors" International Journal of Molecular Sciences 23, no. 20: 12700. https://doi.org/10.3390/ijms232012700