Development of a Hybrid Biomimetic Enamel-Biocomposite Interface and a Study of Its Molecular Features Using Synchrotron Submicron ATR-FTIR Microspectroscopy and Multivariate Analysis Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation Methodology
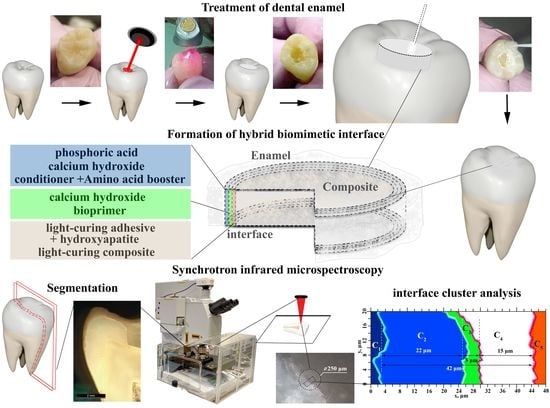
2.1.1. Dental Tissue Samples
2.1.2. Treatment of Dental Tissue
2.1.3. Forming Interfaces
2.1.4. Experiment Design
2.1.5. Sectioning
2.2. Materials
2.2.1. Conditioner
2.2.2. Modified Conditioner with Amino Acids Booster
2.2.3. Bioprimer
2.2.4. Calcium Hydroxide
2.2.5. Modified Adhesive
2.2.6. Nanoscale Carbonate-Substituted Calcium Hydroxyapatite (HAp)
2.3. Microscopy
2.4. Synchrotron FTIR Microspectroscopy
2.5. Multivariate Statistical Analysis
3. Results and Discussion
4. Conclusions
- -
- the use of amino acids and an alkaline solution for pretreatment and biointerface formation results not only in an excess of calcium in the hybrid transition layer but also in the creation of conditions for calcium binding to phosphate complexes (HPO4 and PO4);
- -
- the repeated calcium alkali treatment process can contribute to the formation of conditions where the pretreated enamel surface, after etching, has bonds characteristic of the enamel apatite complex before treatment, and contributes to the formation of bonds with amino acids in the composition of the booster used;
- -
- during diffusion of the amino acid booster conditioner component and the modified HAp adhesive, a structure is formed in the hybrid interface region, which should stabilize the reconstituted crystalline enamel layer.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeRocher, K.A.; Smeets, P.J.M.; Goodge, B.H.; Zachman, M.J.; Balachandran, P.V.; Stegbauer, L.; Cohen, M.J.; Gordon, L.M.; Rondinelli, J.M.; Kourkoutis, L.F.; et al. Chemical Gradients in Human Enamel Crystallites. Nature 2020, 583, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Beniash, E.; Stifler, C.A.; Sun, C.-Y.; Jung, G.S.; Qin, Z.; Buehler, M.J.; Gilbert, P.U.P.A. The Hidden Structure of Human Enamel. Nat. Commun. 2019, 10, 4383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, T.; Takagaki, T.; Hatayama, T.; Nikaido, T.; Tagami, J. Update on Enamel Bonding Strategies. Front. Dent. Med. 2021, 2, 666379. [Google Scholar] [CrossRef]
- Raszewski, Z.; Brząkalski, D.; Jałbrzykowski, M.; Pakuła, D.; Frydrych, M.; Przekop, R.E. Novel Multifunctional Spherosilicate-Based Coupling Agents for Improved Bond Strength and Quality in Restorative Dentistry. Materials 2022, 15, 3451. [Google Scholar] [CrossRef]
- Heintze, S.D.; Loguercio, A.D.; Hanzen, T.A.; Reis, A.; Rousson, V. Clinical Efficacy of Resin-Based Direct Posterior Restorations and Glass-Ionomer Restorations—An Updated Meta-Analysis of Clinical Outcome Parameters. Dent. Mater. 2022, 38, e109–e135. [Google Scholar] [CrossRef]
- Desai, H.; Stewart, C.A.; Finer, Y. Minimally Invasive Therapies for the Management of Dental Caries—A Literature Review. Dent. J. 2021, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Perdigão, J. Current Perspectives on Dental Adhesion: (1) Dentin Adhesion—Not There Yet. Jpn. Dent. Sci. Rev. 2020, 56, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.; Czarnecka, B. Materials for the Direct Restoration of Teeth; Woodhead Publishing: Sawston, UK, 2016; ISBN 978-0-08-100494-4. [Google Scholar]
- Teaford, M.F.; Smith, M.M.; Ferguson, M.W.J. Development, Function and Evolution of Teeth; Cambridge University Press: Cambridge, UK, 2007; ISBN 978-1-139-42922-1. [Google Scholar]
- Wang, L.; Tang, R.; Bonstein, T.; Orme, C.A.; Bush, P.J.; Nancollas, G.H. A New Model for Nanoscale Enamel Dissolution. J. Phys. Chem. B 2005, 109, 999–1005. [Google Scholar] [CrossRef]
- Daher, R.; Krejci, I.; Mekki, M.; Marin, C.; Di Bella, E.; Ardu, S. Effect of Multiple Enamel Surface Treatments on Micro-Shear Bond Strength. Polymers 2021, 13, 3589. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, A.; Ramos, J. Adhesion in Restorative Dentistry; IntechOpen: London, UK, 2016; ISBN 978-953-51-2784-0. [Google Scholar]
- Lempel, E.; Lovász, B.V.; Bihari, E.; Krajczár, K.; Jeges, S.; Tóth, Á.; Szalma, J. Long-Term Clinical Evaluation of Direct Resin Composite Restorations in Vital vs. Endodontically Treated Posterior Teeth—Retrospective Study up to 13 Years. Dent. Mater. 2019, 35, 1308–1318. [Google Scholar] [CrossRef]
- Usha, C.; Ramarao, S.; John, B.M.; Rajesh, P.; Swatha, S. Evaluation of the Shear Bond Strength of Composite Resin to Wet and Dry Enamel Using Dentin Bonding Agents Containing Various Solvents. J. Clin. Diagn. Res. 2017, 11, ZC41–ZC44. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S. Biomimetic Dentistry. J. Oral Res. Rev. 2018, 10, 28–32. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Ren, B.; Wang, Q.; Wu, J.; Yang, N.; Sui, X.; Li, L.; Li, M.; Zhang, X.; et al. Biomimetic Mineralisation Systems for in Situ Enamel Restoration Inspired by Amelogenesis. J. Mater. Sci. Mater. Med. 2021, 32, 115. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Liu, Y.-J.; Zhang, Y.-L.; Zhang, S.-Y.; Han, B.-B.; Chen, F.; Chen, Y.-X. Phosphorylated and Phosphonated Low-Complexity Protein Segments for Biomimetic Mineralization and Repair of Tooth Enamel. Adv. Sci. 2022, 9, 2103829. [Google Scholar] [CrossRef] [PubMed]
- Erceg, I.; Maltar-Strmečki, N.; Jurašin, D.D.; Strasser, V.; Ćurlin, M.; Lyons, D.M.; Radatović, B.; Mlinarić, N.M.; Kralj, D.; Sikirić, M.D. Comparison of the Effect of the Amino Acids on Spontaneous Formation and Transformation of Calcium Phosphates. Crystals 2021, 11, 792. [Google Scholar] [CrossRef]
- Saranya, S.; Samuel Justin, S.J.; Vijay Solomon, R.; Wilson, P. L-Arginine Directed and Ultrasonically Aided Growth of Nanocrystalline Hydroxyapatite Particles with Tunable Morphology. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 270–279. [Google Scholar] [CrossRef]
- Lee, W.H.; Loo, C.Y.; Van, K.L.; Zavgorodniy, A.V.; Rohanizadeh, R. Regulating Protein Adsorption onto Hydroxyapatite: Amino Acid Treatment. Key Eng. Mater. 2012, 493–494, 666–671. [Google Scholar] [CrossRef]
- Tavafoghi, M.; Cerruti, M. The Role of Amino Acids in Hydroxyapatite Mineralization. J. R. Soc. Interface 2016, 13, 20160462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goloshchapov, D.; Kashkarov, V.; Nikitkov, K.; Seredin, P. Investigation of the Effect of Nanocrystalline Calcium Carbonate-Substituted Hydroxyapatite and L-Lysine and L-Arginine Surface Interactions on the Molecular Properties of Dental Biomimetic Composites. Biomimetics 2021, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Comeau, P.; Willett, T. Impact of Side Chain Polarity on Non-Stoichiometric Nano-Hydroxyapatite Surface Functionalization with Amino Acids. Sci. Rep. 2018, 8, 12700. [Google Scholar] [CrossRef]
- Zafar, M.S.; Amin, F.; Fareed, M.A.; Ghabbani, H.; Riaz, S.; Khurshid, Z.; Kumar, N. Biomimetic Aspects of Restorative Dentistry Biomaterials. Biomimetics 2020, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Seredin, P.V.; Goloshchapov, D.L.; Prutskij, T.; Ippolitov, Y.A. Fabrication and Characterisation of Composites Materials Similar Optically and in Composition to Native Dental Tissues. Results Phys. 2017, 7, 1086–1094. [Google Scholar] [CrossRef]
- Lagazzo, A.; Barberis, F.; Carbone, C.; Ramis, G.; Finocchio, E. Molecular Level Interactions in Brushite-Aminoacids Composites. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 721–727. [Google Scholar] [CrossRef]
- Katti, K.S.; Ambre, A.H.; Peterka, N.; Katti, D.R. Use of Unnatural Amino Acids for Design of Novel Organomodified Clays as Components of Nanocomposite Biomaterials. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2010, 368, 1963–1980. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, D.; Ren, H.; Yan, Y.; Li, S. Biological Evaluation of the Modified Nano-Amorphous Phosphate Calcium Doped with Citrate/Poly-Amino Acid Composite as a Potential Candidate for Bone Repair and Reconstruction. J. Mater. Sci. Mater. Med. 2021, 32, 16. [Google Scholar] [CrossRef] [PubMed]
- de Pereira, C.N.B.; Daleprane, B.; de Miranda, G.L.P.; de MagalhãEs, C.S.; Moreira, A.N. Ultramorphology of Pre-Treated Adhesive Interfaces between Self-Adhesive Resin Cement and Tooth Structures. Rev. Odontol. UNESP 2017, 46, 249–254. [Google Scholar] [CrossRef]
- Hattar, S.; Hatamleh, M.M.; Sawair, F.; Al-Rabab’ah, M. Bond Strength of Self-Adhesive Resin Cements to Tooth Structure. Saudi Dent. J. 2015, 27, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Seredin, P.; Goloshchapov, D.; Ippolitov, Y.; Vongsvivut, J. Engineering of a Biomimetic Interface between a Native Dental Tissue and Restorative Composite and Its Study Using Synchrotron FTIR Microscopic Mapping. Int. J. Mol. Sci. 2021, 22, 6510. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Emelyanova, A.; Buylov, N.; Kashkarov, V.; Lukin, A.; Ippolitov, Y.; Khmelevskaia, T.; Mahdy, I.A.; Mahdy, M.A. Engineering of Biomimetic Mineralized Layer Formed on the Surface of Natural Dental Enamel. Results Eng. 2022, 15, 100583. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Emelyanova, A.; Buylov, N.; Barkov, K.; Ippolitov, Y.; Khmelevskaia, T.; Mahdy, I.A.; Mahdy, M.A.; et al. Biomimetic Mineralization of Tooth Enamel Using Nanocrystalline Hydroxyapatite under Various Dental Surface Pretreatment Conditions. Biomimetics 2022, 7, 111. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Y.; Gao, L.; Wu, C.; Chang, J. Synthesis of Artificial Dental Enamel by an Elastin-like Polypeptide Assisted Biomimetic Approach. J. Mater. Chem. B 2018, 6, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Pullano, S.A.; Bianco, M.G.; Greco, M.; Mazzuca, D.; Nisticò, S.P.; Fiorillo, A.S. FT-IR Saliva Analysis for the Diagnosis of Psoriasis: A Pilot Study. Biomed. Signal Process. Control 2022, 74, 103525. [Google Scholar] [CrossRef]
- Calzolari, A.; Pavan, B.; Curtarolo, S.; Buongiorno Nardelli, M.; Fornari, M. Vibrational Spectral Fingerprinting for Chemical Recognition of Biominerals. ChemPhysChem 2020, 21, 770–778. [Google Scholar] [CrossRef]
- Uskoković, V. Visualizing Different Crystalline States during the Infrared Imaging of Calcium Phosphates. Vib. Spectrosc. 2020, 108, 103045. [Google Scholar] [CrossRef] [PubMed]
- Bērziņš, K.; Sutton, J.J.; Loch, C.; Beckett, D.; Wheeler, B.J.; Drummond, B.K.; Fraser-Miller, S.J.; Gordon, K.C. Application of Low-wavenumber Raman Spectroscopy to the Analysis of Human Teeth. J. Raman Spectrosc. 2019, 50, 1375–1387. [Google Scholar] [CrossRef]
- Güler, G.; Vorob’ev, M.M.; Vogel, V.; Mäntele, W. Proteolytically-Induced Changes of Secondary Structural Protein Conformation of Bovine Serum Albumin Monitored by Fourier Transform Infrared (FT-IR) and UV-Circular Dichroism Spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 161, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Titus, J.; Ghimire, H.; Viennois, E.; Merlin, D.; Perera, A.G.U. Protein Secondary Structure Analysis of Dried Blood Serum Using Infrared Spectroscopy to Identify Markers for Colitis Screening. J. Biophotonics 2018, 11, e201700057. [Google Scholar] [CrossRef] [PubMed]
- Pazderka, T.; Kopecký, V. Drop Coating Deposition Raman Spectroscopy of Proteinogenic Amino Acids Compared with Their Solution and Crystalline State. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 207–216. [Google Scholar] [CrossRef]
- Paiva, F.M.; Batista, J.C.; Rêgo, F.S.C.; Lima, J.A.; Freire, P.T.C.; Melo, F.E.A.; Mendes Filho, J.; de Menezes, A.S.; Nogueira, C.E.S. Infrared and Raman Spectroscopy and DFT Calculations of DL Amino Acids: Valine and Lysine Hydrochloride. J. Mol. Struct. 2017, 1127, 419–426. [Google Scholar] [CrossRef]
- Ye, Q.; Spencer, P. Analyses of Material-Tissue Interfaces by Fourier Transform Infrared, Raman Spectroscopy, and Chemometrics. In Material-Tissue Interfacial Phenomena; Elsevier: Amsterdam, The Netherlands, 2017; pp. 231–251. ISBN 978-0-08-100330-5. [Google Scholar]
- Seredin, P.; Goloshchapov, D.; Ippolitov, Y.; Vongsvivut, J. Development of a New Approach to Diagnosis of the Early Fluorosis Forms by Means of FTIR and Raman Microspectroscopy. Sci. Rep. 2020, 10, 20891. [Google Scholar] [CrossRef]
- Desoutter, A.; Slimani, A.; Al-Obaidi, R.; Barthélemi, S.; Cuisinier, F.; Tassery, H.; Salehi, H. Cross Striation in Human Permanent and Deciduous Enamel Measured with Confocal Raman Microscopy. J. Raman Spectrosc. 2019, 50, 548–556. [Google Scholar] [CrossRef]
- Delgado, A.H.; Young, A.M. Modelling ATR-FTIR Spectra of Dental Bonding Systems to Investigate Composition and Polymerisation Kinetics. Materials 2021, 14, 760. [Google Scholar] [CrossRef] [PubMed]
- Tekçe, N.; Tuncer, S.; Demirci, M.; Serim, M.E.; Baydemir, C. The Effect of Different Drinks on the Color Stability of Different Restorative Materials after One Month. Restor. Dent. Endod. 2015, 40, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Seredin, P.; Goloshchapov, D.; Prutskij, T.; Ippolitov, Y. Phase Transformations in a Human Tooth Tissue at the Initial Stage of Caries. PLoS ONE 2015, 10, e0124008. [Google Scholar] [CrossRef] [PubMed]
- Goloshchapov, D.L.; Kashkarov, V.M.; Ippolitov, Y.A.; Prutskij, T.; Seredin, P.V. Early Screening of Dentin Caries Using the Methods of Micro-Raman and Laser-Induced Fluorescence Spectroscopy. Results Phys. 2018, 10, 346–347. [Google Scholar] [CrossRef]
- Goloshchapov, D.L.; Ippolitov, Y.A.; Seredin, P.V. Mechanism of Interaction among Nanocrystalline Carbonate-Substituted Hydroxyapatite and Polar Amino-Acids for the Biomimetic Composite Technology: Spectroscopic and Structural Study. Results Phys. 2020, 18, 103277. [Google Scholar] [CrossRef]
- Fugolin, A.P.; Sundfeld, D.; Ferracane, J.L.; Pfeifer, C.S. Toughening of Dental Composites with Thiourethane-Modified Filler Interfaces. Sci. Rep. 2019, 9, 2286. [Google Scholar] [CrossRef] [Green Version]
- Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Ippolitov, Y.; Vongsvivut, J. The Molecular and Mechanical Characteristics of Biomimetic Composite Dental Materials Composed of Nanocrystalline Hydroxyapatite and Light-Cured Adhesive. Biomimetics 2022, 7, 35. [Google Scholar] [CrossRef]
- Goloshchapov, D.L.; Kashkarov, V.M.; Rumyantseva, N.A.; Seredin, P.V.; Lenshin, A.S.; Agapov, B.L.; Domashevskaya, E.P. Synthesis of Nanocrystalline Hydroxyapatite by Precipitation Using Hen’s Eggshell. Ceram. Int. 2013, 39, 4539–4549. [Google Scholar] [CrossRef]
- Goloshchapov, D.L.; Lenshin, A.S.; Savchenko, D.V.; Seredin, P.V. Importance of Defect Nanocrystalline Calcium Hydroxyapatite Characteristics for Developing the Dental Biomimetic Composites. Results Phys. 2019, 13, 102158. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, S.; Matinlinna, J.P.; Chen, Z.; Pan, H. Insight into Biological Apatite: Physicochemical Properties and Preparation Approaches. BioMed Res. Int. 2013, 2013, 929748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanci, A. Ten Cate’s Oral Histology: Development, Structure, and Function, 8th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013; ISBN 978-0-323-07846-7. [Google Scholar]
- Vongsvivut, J.; Pérez-Guaita, D.; Wood, B.R.; Heraud, P.; Khambatta, K.; Hartnell, D.; Hackett, M.J.; Tobin, M.J. Synchrotron Macro ATR-FTIR Microspectroscopy for High-Resolution Chemical Mapping of Single Cells. Analyst 2019, 144, 3226–3238. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, X.; Parthasarathy, R. Characterization of Interfacial Chemistry of Adhesive/Dentin Bond Using FTIR Chemical Imaging with Univariate and Multivariate Data Processing. J. Biomed. Mater. Res. A 2009, 91, 251–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savić, D.; Joković, N.; Topisirović, L. Multivariate Statistical Methods for Discrimination of Lactobacilli Based on Their FTIR Spectra. Dairy Sci. Technol. 2008, 88, 273–290. [Google Scholar] [CrossRef]
- Matwijczuk, A.; Oniszczuk, T.; Matwijczuk, A.; Chruściel, E.; Kocira, A.; Niemczynowicz, A.; Wójtowicz, A.; Combrzyński, M.; Wiącek, D. Use of FTIR Spectroscopy and Chemometrics with Respect to Storage Conditions of Moldavian Dragonhead Oil. Sustainability 2019, 11, 6414. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Yuan, Y.; Han, S.; Yang, H. Application of Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR) and Principal Component Analysis (PCA) for Quick Identifying of the Bitumen Produced by Different Manufacturers. Road Mater. Pavement Des. 2018, 19, 1940–1949. [Google Scholar] [CrossRef]
- Spencer, P.; Wang, Y.; Katz, J.L.; Misra, A. Physicochemical Interactions at the Dentin/Adhesive Interface Using FTIR Chemical Imaging. J. Biomed. Opt. 2005, 10, 031104. [Google Scholar] [CrossRef] [Green Version]
- Temel, U.B.; Van Ende, A.; Van Meerbeek, B.; Ermis, R.B. Bond Strength and Cement-Tooth Interfacial Characterization of Self-Adhesive Composite Cements. Am. J. Dent. 2017, 30, 205–211. [Google Scholar]
- de Lopes, C.C.A.; Limirio, P.H.J.O.; Novais, V.R.; Dechichi, P. Fourier Transform Infrared Spectroscopy (FTIR) Application Chemical Characterization of Enamel, Dentin and Bone. Appl. Spectrosc. Rev. 2018, 53, 747–769. [Google Scholar] [CrossRef]
- Reyes-Gasga, J.; Martínez-Piñeiro, E.L.; Rodríguez-Álvarez, G.; Tiznado-Orozco, G.E.; García-García, R.; Brès, E.F. XRD and FTIR Crystallinity Indices in Sound Human Tooth Enamel and Synthetic Hydroxyapatite. Mater. Sci. Eng. C 2013, 33, 4568–4574. [Google Scholar] [CrossRef]
- Jegova, G.; Titorenkova, R.; Rashkova, M.; Mihailova, B. Raman and IR Reflection Micro-Spectroscopic Study of Er:YAG Laser Treated Permanent and Deciduous Human Teeth. J. Raman Spectrosc. 2013, 44, 1483–1490. [Google Scholar] [CrossRef]
- Jeon, R.J.; Hellen, A.; Matvienko, A.; Mandelis, A.; Abrams, S.H.; Amaechi, B.T. In Vitro Detection and Quantification of Enamel and Root Caries Using Infrared Photothermal Radiometry and Modulated Luminescence. J. Biomed Opt. 2008, 13, 034025. [Google Scholar] [CrossRef] [PubMed]
- Rey, C.; Marsan, O.; Combes, C.; Drouet, C.; Grossin, D.; Sarda, S. Characterization of Calcium Phosphates Using Vibrational Spectroscopies. In Advances in Calcium Phosphate Biomaterials; Springer Series in Biomaterials Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2014; pp. 229–266. ISBN 978-3-642-53979-4. [Google Scholar]
- Lubarsky, G.V.; D’Sa, R.A.; Deb, S.; Meenan, B.J.; Lemoine, P. The Role of Enamel Proteins in Protecting Mature Human Enamel Against Acidic Environments: A Double Layer Force Spectroscopy Study. Biointerphases 2012, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resende, N.S.; Nele, M.; Salim, V.M.M. Effects of Anion Substitution on the Acid Properties of Hydroxyapatite. Thermochim. Acta 2006, 451, 16–21. [Google Scholar] [CrossRef]
- Fleet, M.E. Infrared Spectra of Carbonate Apatites: Evidence for a Connection between Bone Mineral and Body Fluids. Am. Mineral. 2017, 102, 149–157. [Google Scholar] [CrossRef]
- Spevak, L.; Flach, C.R.; Hunter, T.; Mendelsohn, R.; Boskey, A. Fourier Transform Infrared Spectroscopic Imaging Parameters Describing Acid Phosphate Substitution in Biologic Hydroxyapatite. Calcif. Tissue Int. 2013, 92, 418–428. [Google Scholar] [CrossRef] [Green Version]
- Han, F.; Liang, R.; Xie, H. Effects of Phosphoric Acid Pre-Etching on Chemisorption between Enamel and MDP-Containing Universal Adhesives: Chemical and Morphological Characterization, and Evaluation of Its Potential. ACS Omega 2021, 6, 13182–13191. [Google Scholar] [CrossRef] [PubMed]
- Villegas, M.F.; Garcia-Uriostegui, L.; Rodríguez, O.; Izquierdo-Barba, I.; Salinas, A.J.; Toriz, G.; Vallet-Regí, M.; Delgado, E. Lysine-Grafted MCM-41 Silica as an Antibacterial Biomaterial. Bioengineering 2017, 4, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, B.; Pflüger, F.; Derbel, N.; De Coninck, J.; Ghomi, M. Vibrational Analysis of Amino Acids and Short Peptides in Hydrated Media. VI. Amino Acids with Positively Charged Side Chains: L-Lysine and L-Arginine. J. Phys. Chem. B 2010, 114, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xun, Q.; Liu, S.; Wang, X. Investigation of Ethylene Glycol Monomethyl Ether Soyate as a Biofuel; SAE International: Warrendale, PA, USA, 2015. [Google Scholar]
- Gupta, B.S.; Jelle, B.P.; Gao, T. In Vitro Cell Composition Identification of Wood Decay Fungi by Fourier Transform Infrared Spectroscopy. R. Soc. Open Sci. 2022, 9, 201935. [Google Scholar] [CrossRef] [PubMed]
- Barth, A. The Infrared Absorption of Amino Acid Side Chains. Prog. Biophys. Mol. Biol. 2000, 74, 141–173. [Google Scholar] [CrossRef]
- Barth, A. Infrared Spectroscopy of Proteins. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daood, U.; Swee Heng, C.; Neo Chiew Lian, J.; Fawzy, A.S. In Vitro Analysis of Riboflavin-Modified, Experimental, Two-Step Etch-and-Rinse Dentin Adhesive: Fourier Transform Infrared Spectroscopy and Micro-Raman Studies. Int. J. Oral Sci. 2015, 7, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Parthasarathy, R.; Abedin, F.; Laurence, J.S.; Misra, A.; Spencer, P. Multivariate Analysis of Attenuated Total Reflection Fourier Transform Infrared (ATR FT-IR) Spectroscopic Data to Confirm Phase Partitioning in Methacrylate-Based Dentin Adhesive. Appl. Spectrosc. 2013, 67, 1473–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayyer, M.; Zahid, S.; Hassan, S.H.; Mian, S.A.; Mehmood, S.; Khan, H.A.; Kaleem, M.; Zafar, M.S.; Khan, A.S. Comparative Abrasive Wear Resistance and Surface Analysis of Dental Resin-Based Materials. Eur. J. Dent. 2018, 12, 057–066. [Google Scholar] [CrossRef]
- Khan, A.S.; Khalid, H.; Sarfraz, Z.; Khan, M.; Iqbal, J.; Muhammad, N.; Fareed, M.A.; Rehman, I.U. Vibrational Spectroscopy of Selective Dental Restorative Materials. Appl. Spectrosc. Rev. 2017, 52, 507–540. [Google Scholar] [CrossRef]
- Hędzelek, W.; Marcinkowska, A.; Domka, L.; Wachowiak, R. Infrared Spectroscopic Identification of Chosen Dental Materials and Natural Teeth. Acta Phys. Pol. A 2008, 114, 471–484. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Ippolitov, Y.; Vongsvivut, P. Pathology-Specific Molecular Profiles of Saliva in Patients with Multiple Dental Caries—Potential Application for Predictive, Preventive and Personalised Medical Services. EPMA J. 2018, 9, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Seredin, P.V.; Goloshchapov, D.L.; Gushchin, M.S.; Ippolitov, Y.A.; Prutskij, T. The Importance of the Biomimetic Composites Components for Recreating the Optical Properties and Molecular Composition of Intact Dental Tissues. J. Phys. Conf. Ser. 2017, 917, 042019. [Google Scholar] [CrossRef]
- Kobrina, Y.; Rieppo, L.; Saarakkala, S.; Pulkkinen, H.J.; Tiitu, V.; Valonen, P.; Kiviranta, I.; Jurvelin, J.S.; Isaksson, H. Cluster Analysis of Infrared Spectra Can Differentiate Intact and Repaired Articular Cartilage. Osteoarthr. Cartil. 2013, 21, 462–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Ippolitov, Y.; Ippolitov, I.; Vongsvivut, J. To the Question on the Use of Multivariate Analysis and 2D Visualisation of Synchrotron ATR-FTIR Chemical Imaging Spectral Data in the Diagnostics of Biomimetic Sound Dentin/Dental Composite Interface. Diagnostics 2021, 11, 1294. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Khudyakov, Y.; Ippolitov, I.; Ippolitov, Y.; Vongsvivut, J. Biomimetic Nano-c-HAp Hybrid Layer Engineering and Determination of Mechanisms of Its Integration with Native Hard Dental Tissue. Results Eng. 2021, 11, 100266. [Google Scholar] [CrossRef]
- Ogruc Ildiz, G.; Karadag, A.; Kaygisiz, E.; Fausto, R. PLS-DA Model for the Evaluation of Attention Deficit and Hyperactivity Disorder in Children and Adolescents through Blood Serum FTIR Spectra. Molecules 2021, 26, 3400. [Google Scholar] [CrossRef]
- Babot-Marquillas, C.; Sánchez-Martín, M.-J.; Amigo, J.M.; Yousef, I.; Valido, I.H.; Boada, R.; Valiente, M. Tooth Whitening, Oxidation or Reduction? Study of Physicochemical Alterations in Bovine Enamel Using Synchrotron Based Micro-FTIR. Dent. Mater. 2022, 38, 670–679. [Google Scholar] [CrossRef]
- Drouet, C. Apatite Formation: Why It May Not Work as Planned, and How to Conclusively Identify Apatite Compounds. BioMed Res. Int. 2013, 2013, 490946. [Google Scholar] [CrossRef] [Green Version]
- Diez-García, S.; Sánchez-Martín, M.-J.; Amigo, J.M.; Valiente, M. Combination of Two Synchrotron Radiation-Based Techniques and Chemometrics to Study an Enhanced Natural Remineralization of Enamel. Anal. Chem. 2022, 94, 5359–5366. [Google Scholar] [CrossRef]
- Goloshchapov, D.; Buylov, N.; Emelyanova, A.; Ippolitov, I.; Ippolitov, Y.; Kashkarov, V.; Khudyakov, Y.; Nikitkov, K.; Seredin, P. Raman and XANES Spectroscopic Study of the Influence of Coordination Atomic and Molecular Environments in Biomimetic Composite Materials Integrated with Dental Tissue. Nanomaterials 2021, 11, 3099. [Google Scholar] [CrossRef]
- Konashuk, A.S.; Samoilenko, D.O.; Klyushin, A.Y.; Svirskiy, G.I.; Sakhonenkov, S.S.; Brykalova, X.O.; Kuz’mina, M.A.; Filatova, E.O.; Vinogradov, A.S.; Pavlychev, A.A. Thermal Changes in Young and Mature Bone Nanostructure Probed with Ca 2p Excitations. Biomed. Phys. Eng. Express 2018, 4, 035031. [Google Scholar] [CrossRef]








| Samples | Alkali | Amino Acids Booster in Dental Conditioner | Conditioner Treatment | Bioprimer | BisGMA Adhesive | HAp in BisGMA Adhesive | DyractXP |
|---|---|---|---|---|---|---|---|
| SI | − | − | + | + | + | − | + |
| SII | + | − | + | + | + | − | + |
| SIII | + | + | + | + | − | + | + |
| SIV | ++++ | + | ++ | + | − | + | + |
| Substance/ Material | Spectral Area, cm−1 | Functional (Molecular) Group | References |
|---|---|---|---|
| Enamel | 1620–1700 | Amide I C=O stretching | [64,69] |
| 1550 | Amide II C─N stretching and N─H deformation modes, CNH | [64,69] | |
| 1535 | OH− substituted by CO32− (type A) | [70,71] | |
| 1465 1417 | PO3− substituted by CO32− (type B) | [70,71] | |
| 1130 | HPO42− | [68,72] | |
| 1030 | HPO42− | [64,70,73] | |
| 960 | υ1 PO43− Symmetric stretching | [64] | |
| HAp | 1460 | PO43− substituted by CO32− (type B) | [25,70] |
| 1415 | PO43− substituted by CO32− (type B) | [25,70] | |
| 1092 | υ3 PO43− Antisymmetric stretching | [25] | |
| 1040 | υ3 PO43− Antisymmetric stretching | [37] | |
| 963 | υ1 PO43− Symmetric stretching | [25] | |
| Conditioner + Amino acids booster | 1718 | C=O carbonyl group of AA | [74] |
| 1635 | vas, COO- vas(CN3H+5) | [50] | |
| 1460–1445 | CH2/CH3 | [50] | |
| 1362 | N-Cα-Hα, Cβ-Cα-Hα | [50] | |
| 1226 | NH3+ | [50,75] | |
| 1185 | ρ, NH3+ | [50] | |
| Bioprimer | 1703 | C=O stretching | [76] |
| 1635 | vas(CN3H+5) protein amino acid, arginine | [77,78] | |
| 1454 | –CH2 | [79] | |
| 1320–1298 | [v(C-O)] stretch doublet δ(CH) | [76] | |
| 1164 | C-O-C, δ(CH) | [76] | |
| 1078 | ν(CO) | [79] | |
| 1026 | ν(CC) | [79] | |
| BisGMA Adhesive | 1721 | C=O carbonyl | [80] |
| 1636 | C=C Aliphatic C=C methacrylate groups | [80] | |
| 1609 | phenyl C=C | [80,81] | |
| 1513 | Aromatic C=C | [80] | |
| 1452 | CH2 CH3 | [80,81] | |
| 1402 | =CH2 deformation | [46] | |
| 1320–1290 | [v(C-O)] stretch dublet | [46] | |
| 1242 | Aromatic C–O | [46] | |
| DyractXP commercial material | 1700–1740 | Ester groups -COOCH3 attached to the methacrylate | [82] |
| 1636 | C=C stretching vibration of the methacrylate group | [82,83] | |
| 1608 | C=C in an aromatic ring | [82,83] | |
| 1511 | N–H deformation stretching of urethane dimethacrylate (UDMA) | [82,83] | |
| 1454 | C–H in constituent monomers | [83] | |
| 1297 | symmetric stretching of -O in monomers, Si–O stretching | [83] | |
| 1233 | C–O–C stretching | [82] | |
| 1150 | C–O–C stretching | [82] | |
| 1040–1060 | Si–O from SiO2 -containing fillers | [83,84] |
| Wavenumber, cm−1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PC1 | 961 | 1014 | 1025 | 1718 | ||||||||
| PC2 | 995 | 1059 | 1092 | 1149 | 1248 | 1448 | 1522 | 1538 | 1720 | |||
| Assignment | υ1 PO43− enamel nano-c-HAp | DCPD and β-TCP | υ3 PO43− | the stoichiometric apatites containing HPO4 2− ion | DCPD and β-TCP | υ3 PO43− nano-c-HAp | HPO42− stoichiometric apatites | Aromatic C–O | PO3− substituted by CO32− (type B) CH2−CH3 | Aromatic C=C | δsNH3+ | BIS-GMA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Khydyakov, Y.; Nesterov, D.; Ippolitov, I.; Ippolitov, Y.; Vongsvivut, J. Development of a Hybrid Biomimetic Enamel-Biocomposite Interface and a Study of Its Molecular Features Using Synchrotron Submicron ATR-FTIR Microspectroscopy and Multivariate Analysis Techniques. Int. J. Mol. Sci. 2022, 23, 11699. https://doi.org/10.3390/ijms231911699
Seredin P, Goloshchapov D, Kashkarov V, Khydyakov Y, Nesterov D, Ippolitov I, Ippolitov Y, Vongsvivut J. Development of a Hybrid Biomimetic Enamel-Biocomposite Interface and a Study of Its Molecular Features Using Synchrotron Submicron ATR-FTIR Microspectroscopy and Multivariate Analysis Techniques. International Journal of Molecular Sciences. 2022; 23(19):11699. https://doi.org/10.3390/ijms231911699
Chicago/Turabian StyleSeredin, Pavel, Dmitry Goloshchapov, Vladimir Kashkarov, Yury Khydyakov, Dmitry Nesterov, Ivan Ippolitov, Yuri Ippolitov, and Jitraporn Vongsvivut. 2022. "Development of a Hybrid Biomimetic Enamel-Biocomposite Interface and a Study of Its Molecular Features Using Synchrotron Submicron ATR-FTIR Microspectroscopy and Multivariate Analysis Techniques" International Journal of Molecular Sciences 23, no. 19: 11699. https://doi.org/10.3390/ijms231911699