Use of Steaming Process to Improve Biochemical Activity of Polygonatum sibiricum Polysaccharides against D-Galactose-Induced Memory Impairment in Mice
Abstract
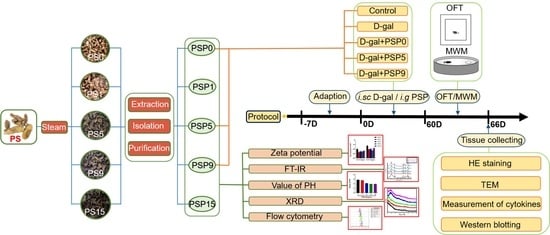
:1. Introduction
2. Results
2.1. Analysis of Physicochemical Properties of PSPs
2.2. Steamed PSPs Promoted Antioxidant Capacity in D-Gal-Treated HT-22 Cells
2.3. Steamed PSPs Improved Spleen Index and Behavioral Functions in D-Gal-Injured Mice
2.4. Steamed PSPs Prevented D-Gal-Induced Morphological Changes of Hippocampal Pyramidal Cells
2.5. Effects of Different Steamed PSPs on HO-1/Nrf2 Signaling Pathway in D-Gal-Induced Mice
2.6. Steamed PSPs Prevented D-Gal-Induced Neuroinflammation in Mice
2.7. Steamed PSPs Improved D-Gal-Induced Synapse Deficits in the Hippocampus
3. Discussion
3.1. The Steaming Process Enhances the Biological Activity of PSP
3.2. Steamed PSPs Ameliorate Behavioral Impairment in D-Gal-Induced Mice
3.3. Steamed PSPs Have Protective Effects against D-Gal-Induced Pyramidal Cell Injury in the Hippocampus
3.4. Steamed PSPs Improve Memory Dysfunction in D-Gal-Induced Mice by Enhancing Antioxidant Capacity
3.5. Steamed PSPs Prevent Synaptic Deficits in D-Gal-Induced Mice
4. Materials and Methods
4.1. Animals and Drugs
4.2. Preparation of PSPs
4.3. Determination of Physicochemical Properties of PSPs
4.3.1. Fourier Transform Infrared Spectrometer (FT-IR)
4.3.2. Zeta Potential Test
4.3.3. pH Value Measurement
4.3.4. X-ray Diffraction (XRD) Analysis
4.4. Cell Culture and Oxidative Capacity Assay
4.5. Animal Model and Treatments
4.6. Morris Water Maze (MWM)
4.7. Open Field Test (OFT)
4.8. Hematoxylin and Eosin Staining (HE Staining)
4.9. Spleen Index
4.10. Transmission Electron Microscope (TEM)
4.11. Determination of Malondialdehyde (MDA) and Cytokine Detection
4.12. Western Blotting
4.13. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, R.; Chan, W.; Mat, W.; Ho, Y.; Yeung, R.K.; Tsang, S.; Xue, H. Antiaging and Anxiolytic Effects of Combinatory Formulas Based on Four Medicinal Herbs. Evid.-Based Complement. Altern. Med. 2017, 2017, 4624069. [Google Scholar] [CrossRef]
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Krueger, K.; Farmer, K.; Heinze, J. The effects of age, rank and neophobia on social learning in horses. Anim. Cogn. 2014, 17, 645–655. [Google Scholar] [CrossRef]
- Mecocci, P.; Boccardi, V.; Cecchetti, R.; Bastiani, P.; Scamosci, M.; Ruggiero, C.; Baroni, M. A Long Journey into Aging, Brain Aging, and Alzheimer’s Disease Following the Oxidative Stress Tracks, Journal of Alzheimer’s disease. JAD 2018, 62, 1319–1335. [Google Scholar] [CrossRef]
- Lisman, J.; Buzsáki, G.; Eichenbaum, H.; Nadel, L.; Ranganath, C.; Redish, A.D. Viewpoints: How the hippocampus contributes to memory, navigation and cognition. Nat. Neurosci. 2017, 20, 1434–1447. [Google Scholar] [CrossRef]
- Voss, J.L.; Bridge, D.J.; Cohen, N.J.; Walker, J.A. A Closer Look at the Hippocampus and Memory. Trends Cogn. Sci. 2017, 21, 577–588. [Google Scholar] [CrossRef]
- Fjell, A.M.; McEvoy, L.; Holland, D.; Dale, A.M.; Walhovd, K.B. What is normal in normal aging? Effects of aging, amyloid and Alzheimer′s disease on the cerebral cortex and the hippocampus. Prog. Neurobiol. 2014, 117, 20–40. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, F.; Wang, Z.; Shen, C.; Zheng, Y.; Ma, F.; Zhu, T.; Chen, L.; Tang, Q.; Zhu, J. Aloin attenuates cognitive impairment and inflammation induced by d-galactose via down-regulating ERK, p38 and NF-κB signaling pathway. Int. Immunopharmacol. 2019, 72, 48–54. [Google Scholar] [CrossRef]
- Zhou, X.-Q.; Yao, Z.-W.; Peng, Y.; Mao, S.-S.; Xu, D.; Qin, X.-F.; Zhang, R.-J. PQQ ameliorates D-galactose induced cognitive impairments by reducing glutamate neurotoxicity via the GSK-3β/Akt signaling pathway in mouse. Sci. Rep. 2018, 8, 8894. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Q.L.; Hou, S.B.; Chen, G. Chemical constituents from the rhizomes of Polygonatum sibiricum Red. and anti-inflammatory activity in RAW264.7 macrophage cells. Nat. Prod. Res. 2019, 33, 2359–2362. [Google Scholar] [CrossRef]
- Liu, N.; Dong, Z.; Zhu, X.; Xu, H.; Zhao, Z. Characterization and protective effect of Polygonatum sibiricum polysaccharide against cyclophosphamide-induced immunosuppression in Balb/c mice. Int. J. Biol. Macromol. 2018, 107 Pt. A, 796–802. [Google Scholar] [CrossRef]
- Li, L.; Thakur, K.; Cao, Y.Y.; Liao, B.Y.; Zhang, J.G.; Wei, Z.J. Anticancerous potential of polysaccharides sequentially extracted from Polygonatum cyrtonema Hua in Human cervical cancer Hela cells. Int. J. Biol. Macromol. 2020, 148, 843–850. [Google Scholar] [CrossRef]
- Xie, Y.; Mu, C.; Kazybay, B.; Sun, Q.; Kutzhanova, A.; Nazarbek, G.; Xu, N.; Nurtay, L.; Wang, Q.; Amin, A.; et al. Network pharmacology and experimental investigation of Rhizoma polygonati extract targeted kinase with herbzyme activity for potent drug delivery. Drug Deliv. 2021, 28, 2187–2197. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, Q.; Xie, Y.; Sun, J.; Hu, J.; Qiu, P.; Cao, W.; Wang, S. Tetrahydroxystilbene glucoside extends mouse life span via upregulating neural klotho and downregulating neural insulin or insulin-like growth factor 1. Neurobiol. Aging 2014, 36, 1462–1470. [Google Scholar] [CrossRef]
- Cheng, R.; Liao, X.; Addou, A.M.; Qian, J.; Wang, S.; Cheng, Z.; Wang, L.; Huang, J. Effects of “nine steaming nine sun-drying” on proximate composition, oil properties and volatile compounds of black sesame seeds. Food Chem. 2021, 344, 128577. [Google Scholar] [CrossRef]
- Zhao, P.; Zhao, C.; Li, X.; Gao, Q.; Huang, L.; Xiao, P.; Gao, W. The genus Polygonatum: A review of ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2018, 214, 274–291. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, J.; Gong, P.; Wu, Y.; Li, H. Effect of steaming process on the structural characteristics and antioxidant activities of polysaccharides from Polygonatum sibiricum rhizomes. Glycoconj. J. 2021, 38, 561–572. [Google Scholar] [CrossRef]
- Shen, F.; Xie, P.; Li, C.; Bian, Z.; Wang, X.; Peng, D.; Zhu, G. Polysaccharides from Polygonatum cyrtonema Hua Reduce Depression-Like Behavior in Mice by Inhibiting Oxidative Stress-Calpain-1-NLRP3 Signaling Axis. Oxidative Med. Cell. Longev. 2022, 2022, 2566917. [Google Scholar] [CrossRef]
- Shen, F.; Song, Z.; Xie, P.; Li, L.; Wang, B.; Peng, D.; Zhu, G. Polygonatum sibiricum polysaccharide prevents depression-like behaviors by reducing oxidative stress, inflammation, and cellular and synaptic damage. J. Ethnopharmacol. 2021, 275, 114164. [Google Scholar] [CrossRef]
- Tsai, S.J.; Chiu, C.P.; Yang, H.T.; Yin, M.C. s-Allyl cysteine, s-ethyl cysteine, and s-propyl cysteine alleviate β-amyloid, glycative, and oxidative injury in brain of mice treated by D-galactose. J. Agric. Food Chem. 2011, 59, 6319–6326. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, J.; Wang, B.; Cheng, Z.; Xu, J.; Gao, W.; Chen, K. Structural characterization and antioxidant activities of Bletilla striata polysaccharide extracted by different methods. Carbohydr. Polym. 2021, 266, 118149. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, H.; Yao, H.; Zhou, J.; Duan, Y.; Ma, H. Comparison of characterization, antioxidant and immunological activities of three polysaccharides from Sagittaria sagittifolia L. Carbohydr. Polym. 2020, 235, 115939. [Google Scholar] [CrossRef]
- Ben Jeddou, K.; Chaari, F.; Maktouf, S.; Nouri-Ellouz, O.; Helbert, C.B.; Ghorbel, R.E. Structural, functional, and antioxidant properties of water-soluble polysaccharides from potatoes peels. Food Chem. 2016, 205, 97–105. [Google Scholar] [CrossRef]
- Yang, B.; Wu, Q.; Luo, Y.; Yang, Q.; Wei, X.; Kan, J. High-pressure ultrasonic-assisted extraction of polysaccharides from Hovenia dulcis: Extraction, structure, antioxidant activity and hypoglycemic. Int. J. Biol. Macromol. 2019, 137, 676–687. [Google Scholar] [CrossRef]
- Song, Z.; Shen, F.; Zhang, Z.; Wu, S.; Zhu, G. Calpain inhibition ameliorates depression-like behaviors by reducing inflammation and promoting synaptic protein expression in the hippocampus. Neuropharmacology 2020, 174, 108175. [Google Scholar] [CrossRef]
- Brands, C.M.; van Boekel, M.A. Reactions of monosaccharides during heating of sugar-casein systems: Building of a reaction network model. J. Agric. Food Chem. 2001, 49, 4667–4675. [Google Scholar] [CrossRef]
- Somoza, V. Five years of research on health risks and benefits of Maillard reaction products: An update. Mol. Nutr. Food Res. 2005, 49, 663–672. [Google Scholar] [CrossRef]
- Zhang, H.; Zhong, W.; Wang, M.I.L.A.; Zhao, X. Effect of acupuncture on SIRT3/PGC-1 α protein expression in hippocampus of SAMP8 mice. Lishizhen Med. Mater. Med. Res. 2021, 32, 2036–2041. [Google Scholar]
- Cui, X.; Wang, S.; Cao, H.; Guo, H.; Li, Y.; Xu, F.; Zheng, M.; Xi, X.; Han, C. A Review: The Bioactivities and Pharmacological Applications of Polygonatum sibiricum polysaccharides. Molecules 2018, 23, 1170. [Google Scholar] [CrossRef]
- Zheng, S. Protective effect of Polygonatum sibiricum Polysaccharide on D-galactose-induced aging rats model. Sci. Rep. 2020, 10, 2246. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Zhao, H.; Qi, C.; Ji, X.; Yan, H.; Cui, R.; Zhang, G.; Kang, Y.; Shi, G. Pentoxifylline Enhances Antioxidative Capability and Promotes Mitochondrial Biogenesis in D-Galactose-Induced Aging Mice by Increasing Nrf2 and PGC-1α through the cAMP-CREB Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 6695613. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, B.; Huang, J.; Li, W.; Yi, P.; Yi, M.; Peng, W. Identification of the protective effect of Polygonatum sibiricum polysaccharide on d-galactose-induced brain ageing in mice by the systematic characterization of a circular RNA-associated ceRNA network. Pharm. Biol. 2021, 59, 345–364. [Google Scholar] [CrossRef]
- Burke, S.N.; Barnes, C.A. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 2006, 7, 30–40. [Google Scholar] [CrossRef]
- Kesner, R.P.; Rolls, E.T. A computational theory of hippocampal function, and tests of the theory: New developments. Neurosci. Biobehav. Rev. 2015, 48, 92–147. [Google Scholar] [CrossRef]
- Jeltsch, H.; Bertrand, F.; Lazarus, C.; Cassel, J.-C. Cognitive Performances and Locomotor Activity Following Dentate Granule Cell Damage in Rats: Role of Lesion Extent and Type of Memory Tested. Neurobiol. Learn. Mem. 2001, 76, 81–105. [Google Scholar] [CrossRef]
- van Muiswinkel, F.L.; Kuiperij, H.B. The Nrf2-ARE Signalling pathway: Promising drug target to combat oxidative stress in neurodegenerative disorders, Current drug targets. CNS Neurol. Disord. 2005, 4, 267–281. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Zhu, X.; Sun, X.; Feng, D.; Li, J.; Zhang, M.; Zhu, Z.Z. Methylallyl sulfone attenuates inflammation, oxidative stress and lung injury induced by cigarette smoke extract in mice and RAW264.7 cells. Int. Immunopharmacol. 2018, 59, 369–374. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.-S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Napolioni, V.; MacMurray, J. Infectious diseases, IL6 -174G>C polymorphism, and human development. Brain Behav. Immun. 2016, 51, 196–203. [Google Scholar] [CrossRef]
- Elzi, D.J.; Song, M.; Shiio, Y. Role of galactose in cellular senescence. Exp. Gerontol. 2016, 73, 1–4. [Google Scholar] [CrossRef]
- Toscano, E.C.D.; Vieira, É.L.M.; Dias, B.B.R.; Caliari, M.V.; Gonçalves, A.P.; Giannetti, A.V.; Siqueira, J.M.; Suemoto, C.K.; Leite, R.E.P.; Nitrini, R.; et al. NLRP3 and NLRP1 inflammasomes are up-regulated in patients with mesial temporal lobe epilepsy and may contribute to overexpression of caspase-1 and IL-β in sclerotic hippocampi. Brain Res. 2021, 1752, 147230. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, R.; Zhou, H.; Wang, L.; Wang, R.; Li, H.; Tan, B.; Wu, Q.; Xu, X.; Cui, L.; et al. β-hydroxybutyrate Alleviates Learning and Memory Impairment Through the SIRT1 Pathway in D-Galactose-Injured Mice. Front. Pharmacol. 2021, 12, 751028. [Google Scholar] [CrossRef] [PubMed]
- Ouda, L.; Profant, O.; Syka, J. Age-related changes in the central auditory system. Cell Tissue Res. 2015, 361, 337–358. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.-M.; Herrup, K. Genomic integrity and the ageing brain. Nat. Rev. Neurosci. 2015, 16, 672–684. [Google Scholar] [CrossRef]
- Martínez-Cué, C.; Rueda, N. Cellular Senescence in Neurodegenerative Diseases. Front. Cell. Neurosci. 2020, 14, 16. [Google Scholar] [CrossRef]
- Lu, S.; Zhou, J.; Yang, C.; Zhang, X.; Shi, Y.; Liu, J.; Yan, X.; Liang, J.; Liu, X.; Luo, L.; et al. γ-Glutamylcysteine ameliorates d-gal-induced senescence in PC12 cells and mice via activating AMPK and SIRT1. Food Funct. 2022, 13, 7560–7571. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, Z.; Li, C.; Peng, D.; Wang, X.; Zhu, G. Use of Steaming Process to Improve Biochemical Activity of Polygonatum sibiricum Polysaccharides against D-Galactose-Induced Memory Impairment in Mice. Int. J. Mol. Sci. 2022, 23, 11220. https://doi.org/10.3390/ijms231911220
Bian Z, Li C, Peng D, Wang X, Zhu G. Use of Steaming Process to Improve Biochemical Activity of Polygonatum sibiricum Polysaccharides against D-Galactose-Induced Memory Impairment in Mice. International Journal of Molecular Sciences. 2022; 23(19):11220. https://doi.org/10.3390/ijms231911220
Chicago/Turabian StyleBian, Zhijuan, Congting Li, Daiyin Peng, Xuncui Wang, and Guoqi Zhu. 2022. "Use of Steaming Process to Improve Biochemical Activity of Polygonatum sibiricum Polysaccharides against D-Galactose-Induced Memory Impairment in Mice" International Journal of Molecular Sciences 23, no. 19: 11220. https://doi.org/10.3390/ijms231911220