Cryptotanshinone Alleviates Oxidative Stress and Reduces the Level of Abnormally Aggregated Protein in Caenorhabditis elegans AD Models
Abstract
:1. Introduction
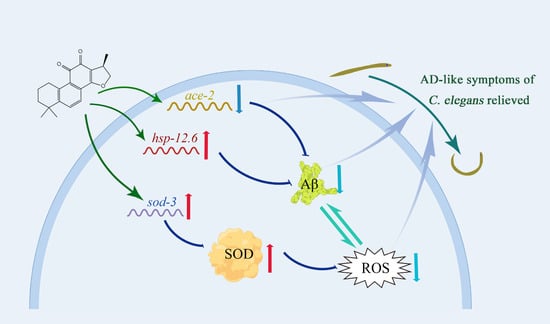
2. Results
2.1. Cryptotanshinone Delayed Progressive Paralysis of AD C. elegans and Decreased the Ratio of Exogenous Serotonin-Induced Acute Paralysis
2.2. Cryptotanshinone Decreased the Level of Aβ Monomers and Oligomers in the Transgenic C. elegans
2.3. Cryptotanshinone Upregulated hsp-16.2 Expression and Reduces Abnormal Polyglutamine Protein Aggregation Levels in Nematodes
2.4. Cryptotanshinone Promoted the Activity of SOD and Alleviated Oxidative Stress in C. elegans
2.5. Cryptotanshinone Upregulated the Expression of Superoxide Dismutase Gene sod-3 and Downregulated the Expression of Cholinesterase Gene ace-2
3. Discussion
4. Materials and Methods
4.1. Strains and Maintenance
4.2. Isolation of Cryptotanshinone from Salvia Castanea and Treatment
4.3. Paralysis Assay
4.4. Sensitivity Assay of Exogenous Serotonin
4.5. Fluorescence Staining of Aβ Deposits
4.6. Western Blotting
4.7. Measurement of ROS Level and SOD Activity in CL4176 Nematodes
4.8. In Vitro Antioxidant Assay
4.9. Subcellular Localization Assay of DAF-16
4.10. Quantitative Analysis of myo-3p::GFP, Q40::YFP, hsp-16.2p::GFP, sod-3p::GFP, Gst-4p::GFP
4.11. Analysis of the Expression Levels of Related Genes
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Association. 2016 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2016, 12, 459–509. [Google Scholar] [CrossRef]
- Qiu, C.; Kivipelto, M.; von Strauss, E. Epidemiology of Alzheimer’s Disease: Occurrence, Determinants, and Strategies toward Intervention. Dialogues Clin. Neurosci. 2009, 11, 111–128. [Google Scholar] [PubMed]
- Mancuso, C.; Bates, T.E.; Butterfield, D.A.; Calafato, S.; Cornelius, C.; de Lorenzo, A.; Kostova, A.T.D.; Calabrese, V. Natural Antioxidants in Alzheimer’s Disease. Expert Opin. Investig. Drugs 2007, 16, 1921–1931. [Google Scholar] [CrossRef]
- Katzman, R.; Hill, L.R.; Yu, E.S.; Wang, Z.Y.; Booth, A.; Salmon, D.P.; Liu, W.T.; Qu, G.Y.; Zhang, M. The Malignancy of Dementia. Predictors of Mortality in Clinically Diagnosed Dementia in a Population Survey of Shanghai, China. Arch. Neurol. 1994, 51, 1220. [Google Scholar] [CrossRef] [PubMed]
- Weiler, P.G. The Public Health Impact of Alzheimer’s Disease. Am. J. Public Health 1987, 77, 1157–1158. [Google Scholar] [CrossRef]
- Li, Y.; Guan, S.; Liu, C.; Chen, X.; Zhu, Y.; Xie, Y.; Wang, J.; Ji, X.; Li, L.; Li, Z.; et al. Neuroprotective Effects of Coptis Chinensis Franch Polysaccharide on Amyloid-Beta (Aβ)-Induced Toxicity in a Transgenic Caenorhabditis elegans Model of Alzheimer’s Disease (AD). Int. J. Biol. Macromol. 2018, 113, 991–995. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s Disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s Disease. Nat. Rev. Dis. Primers 2015, 1, 15056. [Google Scholar] [CrossRef]
- Xu, W.; Tan, L.; Wang, H.F.; Jiang, T.; Tan, M.S.; Tan, L.; Zhao, Q.F.; Li, J.Q.; Wang, J.; Yu, J.T. Meta-Analysis of Modifiable Risk Factors for Alzheimer’s Disease. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1299–1306. [Google Scholar] [CrossRef]
- Tam, K.; Ju, Y. Pathological Mechanisms and Therapeutic Strategies for Alzheimer’s Disease. Neural Regen. Res. 2022, 17, 543. [Google Scholar] [CrossRef]
- Forloni, G.; Balducci, C. Alzheimer’s Disease, Oligomers, and Inflammation. J. Alzheimer’s Dis. 2018, 62, 1261–1276. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, W.; Zhao, M.; Ma, L.; Jiang, X.; Pei, H.; Cao, Y.; Li, H. Interaction between Aβ and Tau in the Pathogenesis of Alzheimer’s Disease. Int. J. Biol. Sci. 2021, 17, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Hayden, E.Y.; Teplow, D.B. Amyloid β-Protein Oligomers and Alzheimer’s Disease. Alzheimer’s Res. Ther. 2013, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.C.; Nam, E.; Lee, H.J.; Savelieff, M.G.; Lim, M.H. Towards an Understanding of Amyloid-β Oligomers: Characterization, Toxicity Mechanisms, and Inhibitors. Chem. Soc. Rev. 2017, 46, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Inagaki, Y.; Liu, Y. Research Progresses on Flavonoids Isolated from Traditional Chinese Medicine in Treatment of Alzheimer’s Disease. Intractable Rare Dis. Res. 2013, 2, 3–10. [Google Scholar] [CrossRef]
- Cummings, J.L.; Morstorf, T.; Zhong, K. Alzheimer’s Disease Drug-Development Pipeline: Few Candidates, Frequent Failures. Alzheimer’s Res. Ther. 2014, 6, 37. [Google Scholar] [CrossRef]
- Cogliati, S.; Clementi, V.; Francisco, M.; Crespo, C.; Argañaraz, F.; Grau, R.; Subtilis, B. Delays Neurodegeneration and Behavioral Impairment in the Alzheimer’s Disease Model Caenorhabditis elegans. J. Alzheimers Dis. 2020, 73, 1035–1052. [Google Scholar] [CrossRef]
- Bolognesi, M.L.; Matera, R.; Minarini, A.; Rosini, M.; Melchiorre, C. Alzheimer’s Disease: New Approaches to Drug Discovery. Curr. Opin. Chem. Biol. 2009, 13, 303–308. [Google Scholar] [CrossRef]
- Cavalli, A.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. Multi-Target-Directed Ligands to Combat Neurodegenerative Diseases. J. Med. Chem. 2008, 51, 347–372. [Google Scholar] [CrossRef]
- Wu, Y.H.; Wu, Y.R.; Li, B.; Yan, Z.Y. Cryptotanshinone: A Review of Its Pharmacology Activities and Molecular Mechanisms. Fitoterapia 2020, 145, 104633. [Google Scholar] [CrossRef]
- Ham, T.J.V. Caenorhabditis elegans Models for Protein Misfolding Diseases; University of Groningen: Groningen, The Netherlands, 2009. [Google Scholar]
- Ruszkiewicz Joanna, A.; Pinkas, A.; Miah, M.R.; Weitz, R.L.; Lawes, M.J.A.; Akinyemi, A.J.; Ijomone, O.M.; Aschner, M. C. elegans as a Model in Developmental Neurotoxicology. FASEB J. 2022, 36, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.-H.; Noh, M.Y.; Kim, S.H. Amyloid-Beta-Induced Neurotoxicity Is Reduced by Inhibition of Glycogen Synthase Kinase-3. Brain Res. 2008, 1188, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Huang, X.B.; Wan, Q.L.; Ding, A.J.; Yang, Z.L.; Qiu, M.H.; Sun, H.Y.; Qi, S.H.; Luo, H.R. Otophylloside B Protects Against Aβ Toxicity in Caenorhabditis elegans Models of Alzheimer’s Disease. Nat. Prod. Bioprospecting 2017, 7, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Kumsta, C.; Chang, J.T.; Schmalz, J.; Hansen, M. Hormetic Heat Stress and HSF-1 Induce Autophagy to Improve Survival and Proteostasis in C. elegans. Nat. Commun. 2017, 8, 14337. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhang, X.; Su, Z.; Xiao, J.; Lv, M.; Cao, Y.; Chen, Y. Carnosol Improved Lifespan and Healthspan by Promoting Antioxidant Capacity in Caenorhabditis elegans. Oxidative Med. Cell. Longev. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Li, H.; Yu, X.; Li, C.; Ma, L.; Zhao, Z.; Guan, S.; Wang, L. Caffeic Acid Protects against Aβ Toxicity and Prolongs Lifespan in Caenorhabditis elegans Models. Food Funct. 2021, 12, 1219–1231. [Google Scholar] [CrossRef]
- William, R. Markesbery Oxidative Stress Hypothesis in Alzheimer’s Disease. Free. Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X. Antioxidant Therapies for Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2012, 2012, 1–17. [Google Scholar] [CrossRef]
- McElwee, J.; Bubb, K.; Thomas, J.H. Transcriptional Outputs of the Caenorhabditis elegans Forkhead Protein DAF-16. Aging Cell 2003, 2, 111–121. [Google Scholar] [CrossRef]
- Link, P.; Roth, K.; Sporer, F.; Wink, M. Carlina acaulis Exhibits Antioxidant Activity and Counteracts Aβ Toxicity in Caenorhabditis elegans. Molecules 2016, 21, 871. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Xu, H.; Liu, L.; Ohulchanskyy, T.Y.; Qu, J. Optical Imaging of Beta-Amyloid Plaques in Alzheimer’s Disease. Biosensors 2021, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Cleary, J.P.; Walsh, D.M.; Hofmeister, J.J.; Shankar, G.M.; Kuskowski, M.A.; Selkoe, D.J.; Ashe, K.H. Natural Oligomers of the Amyloid-β Protein Specifically Disrupt Cognitive Function. Nat. Neurosci. 2005, 8, 79–84. [Google Scholar] [CrossRef]
- Mei, Z.; Zhang, F.; Tao, L.; Zheng, W.; Cao, Y.; Wang, Z.; Tang, S.; Le, K.; Chen, S.; Pi, R.; et al. Cryptotanshinone, a Compound from Salvia Miltiorrhiza Modulates Amyloid Precursor Protein Metabolism and Attenuates β-Amyloid Deposition through Upregulating α-Secretase in Vivo and in Vitro. Neurosci. Lett. 2009, 452, 90–95. [Google Scholar] [CrossRef]
- Mei, Z.; Situ, B.; Tan, X.; Zheng, S.; Zhang, F.; Yan, P.; Liu, P. Cryptotanshinione Upregulates α-Secretase by Activation PI3K Pathway in Cortical Neurons. Brain Res. 2010, 1348, 165–173. [Google Scholar] [CrossRef]
- Durairajan, S.S.K.; Liu, L.F.; Lu, J.H.; Koo, I.; Maruyama, K.; Chung, S.K.; Huang, J.D.; Li, M. Stimulation of Non-Amyloidogenic Processing of Amyloid-β Protein Precursor by Cryptotanshinone Involves Activation and Translocation of ADAM10 and PKC-α. J. Alzheimers Dis. 2011, 25, 245–262. [Google Scholar] [CrossRef]
- Soto, C. Unfolding the Role of Protein Misfolding in Neurodegenerative Diseases. Nat. Rev. Neurosci. 2003, 4, 49–60. [Google Scholar] [CrossRef]
- Zhi, D.; Yang, W.; Yue, J.; Xu, S.; Ma, W.; Zhao, C.; Wang, X.; Wang, D. HSF-1 Mediated Combined Ginsenosides Ameliorating Alzheimer’s Disease like Symptoms in Caernorhabditis Elegans. Nutr. Neurosci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, E.; Wang, R.; Muhammad, F.; Li, T.; Yue, J.; Zhou, Y.; Zhi, D.; Li, H. Ursolic Acid Ameliorates Amyloid β-Induced Pathological Symptoms in Caenorhabditis elegans by Activating the Proteasome. NeuroToxicology 2022, 88, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yi, K.; Zhao, Y. Fucoidan Inhibits Amyloid-β-Induced Toxicity in Transgenic Caenorhabditis elegans by Reducing the Accumulation of Amyloid-β and Decreasing the Production of Reactive Oxygen Species. Food Funct. 2018, 9, 552–560. [Google Scholar] [CrossRef]
- Fonte, V.; Kipp, D.R.; Yerg, J.; Merin, D.; Forrestal, M.; Wagner, E.; Roberts, C.M.; Link, C.D. Suppression of in Vivo β-Amyloid Peptide Toxicity by Overexpression of the HSP-16.2 Small Chaperone Protein. J. Biol. Chem. 2008, 283, 784–791. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.; Hsin, H.; Libina, N.; Kenyon, C. Regulation of the Caenorhabditis elegans Longevity Protein DAF-16 by Insulin/IGF-1 and Germline Signaling. Nat. Genet. 2001, 28, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Kennedy, S.; Tolonen, A.C.; Ruvkun, G. DAF-16 Target Genes That Control C. elegans Life-Span and Metabolism. Science 2003, 300, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Su, Z.; Luo, J.; Jiang, L.; Shen, S.; Zheng, W.; Gu, W.; Cao, Y.; Chen, Y. Polysaccharide Extracted from the Leaves of Cyclocarya Paliurus (Batal.) Iljinskaja Enhanced Stress Resistance in Caenorhabditis elegans via Skn-1 and Hsf-1. Int. J. Biol. Macromol. 2020, 143, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, J.; Li, T.; Liu, R.H. Blueberry Extract Promotes Longevity and Stress Tolerance via DAF-16 in Caenorhabditis elegans. Food Funct. 2011, 9, 5273–5282. [Google Scholar] [CrossRef]
- Murphy, C.T.; McCarroll, S.A.; Bargmann, C.I.; Fraser, A.; Kamath, R.S.; Ahringer, J.; Li, H.; Kenyon, C. Genes That Act Downstream of DAF-16 to Influence the Lifespan of Caenorhabditis elegans. Nature 2003, 424, 277–283. [Google Scholar] [CrossRef]
- Inestrosa, N.C.; Alvarez, A.; Pérez, C.A.; Moreno, R.D.; Vicente, M.; Linker, C.; Casanueva, O.I.; Soto, C.; Garrido, J. Acetylcholinesterase Accelerates Assembly of Amyloid-β-Peptides into Alzheimer’s Fibrils: Possible Role of the Peripheral Site of the Enzyme. Neuron 1996, 16, 881–891. [Google Scholar] [CrossRef]
- Hu, W.; Gray, N.W.; Brimijoin, S. Amyloid-Beta Increases Acetylcholinesterase Expression in Neuroblastoma Cells by Reducing Enzyme Degradation. J. Neurochem. 2003, 86, 470–478. [Google Scholar] [CrossRef]
- Rees, T. Acetylcholinesterase Promotes Beta-Amyloid Plaques in Cerebral Cortex. Neurobiol. Aging 2003, 24, 777–787. [Google Scholar] [CrossRef]
- Xin, L.; Yamujala, R.; Wang, Y.H.; Wang, H.; Wu, W.H.; Lawton, M.; Long, C.; Di, R. Acetylcholineestarase-Inhibiting Alkaloids from Lycoris radiata Delay Paralysis of Amyloid Beta-Expressing Transgenic, C. elegans CL4176. PLoS ONE 2013, 8, e63874. [Google Scholar] [CrossRef]
- Wong, K.K.K.; Ngo, J.C.K.; Liu, S.; Lin, H.; Hu, C.; Shaw, P.C.; Wan, D.C.C. Interaction Study of Two Diterpenes, Cryptotanshinone and Dihydrotanshinone, to Human Acetylcholinesterase and Butyrylcholinesterase by Molecular Docking and Kinetic Analysis. Chem. Biol. Interact. 2010, 187, 335–339. [Google Scholar] [CrossRef]
- Zhu, S.; Li, H.; Dong, J.; Yang, W.; Liu, T.; Wang, Y.; Wang, X.; Wang, M.; Zhi, D. Rose Essential Oil Delayed Alzheimer’s Disease-Like Symptoms by SKN-1 Pathway in C. elegans. J. Agric. Food Chem. 2017, 65, 8855–8865. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhou, Y.; Zhao, L.; Wang, C.; Ma, W.; Ge, G.; Wang, Y.; Ullah, I.; Muhammad, F.; Alwayli, D.; et al. Ferulic Acid Delayed Amyloid β-Induced Pathological Symptoms by Autophagy Pathway via a Fasting-like Effect in Caenorhabditis elegans. Food Chem. Toxicol. 2020, 146, 111808. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Duan, Z.; Wang, Y.; Wang, M.; Liu, Y.; Wang, X.; Li, H. Protective Effect of Terminalia Chebula Retz. Extract against Aβ Aggregation and Aβ-Induced Toxicity in Caenorhabditis elegans. J. Ethnopharmacol. 2021, 268, 113640. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.F.; Tomidokoro, Y.; Ghiso, J.A.; Walker, L.C. SDS-PAGE/Immunoblot Detection of AΒ Multimers in Human Cortical Tissue Homogenates Using Antigen-Epitope Retrieval. J. Vis. Exp. 2010, e1916. [Google Scholar] [CrossRef]
- Jeong, D.E.; Lee, Y.; Lee, S.J.V. Western Blot Analysis of C. elegans Proteins. In Hypoxia; Huang, L.E., Ed.; Humana Press: New York, NY, USA, 2018; Volume 1742, pp. 213–225. ISBN 978-1-4939-7664-5. [Google Scholar]
- Wang, J.; Deng, N.; Wang, H.; Li, T.; Chen, L.; Zheng, B.; Liu, R.H. Effects of Orange Extracts on Longevity, Healthspan, and Stress Resistance in Caenorhabditis elegans. Molecules 2020, 25, 351. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Zhou, L.; Jiao, Y.; Bai, S.; Wang, L.; Ma, J.; Fu, X. Ingredients in Zijuan Pu’er Tea Extract Alleviate β-Amyloid Peptide Toxicity in a Caenorhabditis elegans Model of Alzheimer’s Disease Likely through DAF-16. Molecules 2019, 24, 729. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, Z.; Butko, P.; Christen, Y.; Lambert, M.P.; Klein, W.L.; Link, C.D.; Luo, Y. Amyloid-β-Induced Pathological Behaviors Are Suppressed by Ginkgo Biloba Extract EGb 761 and Ginkgolides in Transgenic Caenorhabditis elegans. J. Neurosci. 2006, 26, 13102–13113. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Wang, D.; Yang, W.; Duan, Z.; Zhu, S.; Dong, J.; Wang, N.; Wang, N.; Fei, D.; Zhang, Z.; et al. Dianxianning Improved Amyloid β-Induced Pathological Characteristics Partially through DAF-2/DAF-16 Insulin like Pathway in Transgenic, C. elegans. Sci. Rep. 2017, 7, 11408. [Google Scholar] [CrossRef]
- Jagota, S.; Rajadas, J. Effect of Phenolic Compounds Against Aβ Aggregation and Aβ-Induced Toxicity in Transgenic, C. elegans. FASEB J. 2022, 36, 40–48. [Google Scholar] [CrossRef]










| Genes | Primers | |
|---|---|---|
| Aβ | F:5′-CCGACATGACTCAGGATATGAAGT-3′ | R:5′-CACCATGAGTCCAATGATTGCA-3′ |
| ace-1 | F:5′-AGTGGGCTCCTGTTCGAGAA-3′ | R:5′-CCAATAGAAAATCACCATCGACAA-3′ |
| ace-2 | F:5′-CAATAATCAACTCATGGGCATCA-3′ | R:5′-TTTTCGCGAGACGAAACGA-3′ |
| bec-1 | F:5′-ACGAGCTTCATTCGCTGGAA-3′ | R:5′-TTCGTGATGTTGTACGCCGA-3′ |
| daf-16 | F:5′-ACCGTTGGTCAAATGCTTGC-3′ | R:5′-TGGCTTCTTACGACAACGCT-3′ |
| hsf-1 | F:5′-ATGCAGCCAGGATTGTCGAA-3′ | R:5′-GCACGTTTTGAGTTGGGTCC-3′ |
| skn-1 | F:5′-GAGAGAAGGGCACACGACAA-3′ | R:5′-TCGAGCATTCTCTTCGGCAG-3′ |
| gst-4 | F:5′-GCTGAAGCCAACGACTCCAT-3′ | R:5′-GACCGAATTGTTCTCCATCGA-3′ |
| sod-1 | F:5′-CGTAGGCGATCTAGGAAATGTG-3′ | R:5′-AACAACCATAGATCGGCCAACG-3′ |
| sod-2 | F:5′-AGCTTTCGGCATCAACTGTC-3′ | R:5′-AAGTCCAGTTGTTGCCTCAAGT-3′ |
| sod-3 | F:5′-TTCAAAGGAGCTGATGGACACT-3′ | R:5′-AAGTGGGACCATTCCTTCCAA-3′ |
| sod-4 | F:5′-GTTGTCTAAGTGCTGGTGG-3′ | R:5′-TTCCACATGCAAGTCGGCT-3′ |
| sod-5 | F:5′-GCAAAATGAATCATGGAGGAAG-3′ | R:5′-AAGATCATCTCGATCGACGTGG-3′ |
| tnfaip1 | F:5′-CCAGAAGAATCCCCATACGA-3′ | R:5′-TCCTCCTCCAACTTTTCCAAA-3′ |
| tnfaip | F:5′-TCCCCATACGAAACAACACA-3′ | R:5′-CTCCTCCCAGCTTTTCCACAA-3′ |
| actin | F:5′-CCACGTCATCAAGGAGTCAT-3′ | R:5′-GGAAGCGTAGAGGGAGAGGA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, W.-B.; Zhang, Z.-P.; Bai, X.; Wang, S.-S.; Chen, X.-H.; Liu, X.; Su, P.-J.; Zhi, D.-J.; Fei, D.-Q.; Zhang, Z.-X.; et al. Cryptotanshinone Alleviates Oxidative Stress and Reduces the Level of Abnormally Aggregated Protein in Caenorhabditis elegans AD Models. Int. J. Mol. Sci. 2022, 23, 10030. https://doi.org/10.3390/ijms231710030
Cui W-B, Zhang Z-P, Bai X, Wang S-S, Chen X-H, Liu X, Su P-J, Zhi D-J, Fei D-Q, Zhang Z-X, et al. Cryptotanshinone Alleviates Oxidative Stress and Reduces the Level of Abnormally Aggregated Protein in Caenorhabditis elegans AD Models. International Journal of Molecular Sciences. 2022; 23(17):10030. https://doi.org/10.3390/ijms231710030
Chicago/Turabian StyleCui, Wen-Bo, Zong-Ping Zhang, Xue Bai, Shan-Shan Wang, Xiao-Han Chen, Xu Liu, Pan-Jie Su, De-Juan Zhi, Dong-Qing Fei, Zhan-Xin Zhang, and et al. 2022. "Cryptotanshinone Alleviates Oxidative Stress and Reduces the Level of Abnormally Aggregated Protein in Caenorhabditis elegans AD Models" International Journal of Molecular Sciences 23, no. 17: 10030. https://doi.org/10.3390/ijms231710030