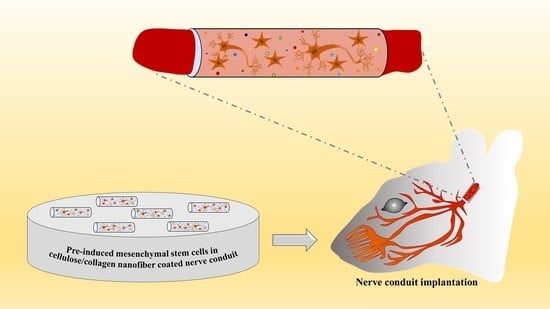
Effect of Pre-Induced Mesenchymal Stem Cell-Coated Cellulose/Collagen Nanofibrous Nerve Conduit on Regeneration of Transected Facial Nerve
Abstract
:1. Introduction
2. Results
2.1. In Vitro Study
2.2. In Vivo Study
2.2.1. Recovery of Vibrissa Fibrillation
2.2.2. Macroscopic Observation
2.2.3. Electrophysiological Studies
2.2.4. Histopathological Studies
3. Discussion
4. Materials and Methods
4.1. Fabrication of the Nerve Conduit
4.2. Scanning Electron Microscopy of the Cellulose/Collagen Nanofiber Nerve Conduit
4.3. Cell Culture and Neuronal Pre-Induction
4.4. Real-Time Polymerase Chain Reaction (PCR)
4.5. Immunocytochemistry
4.6. In Vivo Study
4.6.1. Nerve Conduit Application in a Rat Facial Nerve Transection Model
4.6.2. Evaluation of Vibrissae Fibrillation
4.6.3. Measurement of Threshold of Electrically Stimulated Muscle Action Potential
4.6.4. Histological Examination Using Hematoxylin and Eosin (H&E), Luxol Fast Blue, and Immunohistochemical Staining for Neurofilament and S-100
Tissue Processing and Histochemical Analysis
4.7. Immunohistochemical Analysis
Ultrastructural Findings Using Transmission Electronmicroscopy
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beris, A.; Gkiatas, I.; Gelalis, I.; Papadopoulos, D.; Kostas-Agnantis, I. Current concepts in peripheral nerve surgery. Eur. J. Orthop. Surg. Traumatol. 2019, 29, 263–269. [Google Scholar] [CrossRef]
- Farzamfar, S.; Naseri-Nosar, M.; Vaez, A.; Esmaeilpour, F.; Ehterami, A.; Sahrapeyma, H.; Samadian, H.; Hamidieh, A.-A.; Ghorbani, S.; Goodarzi, A. Neural tissue regeneration by a gabapentin-loaded cellulose acetate/gelatin wet-electrospun scaffold. Cellulose 2018, 25, 1229–1238. [Google Scholar] [CrossRef]
- Frost, H.K.; Andersson, T.; Johansson, S.; Englund-Johansson, U.; Ekström, P.; Dahlin, L.B.; Johansson, F. Electrospun nerve guide conduits have the potential to bridge peripheral nerve injuries in vivo. Sci. Rep. 2018, 8, 16716. [Google Scholar] [CrossRef] [Green Version]
- Urrutia, D.N.; Caviedes, P.; Mardones, R.; Minguell, J.J.; Vega-Letter, A.M.; Jofre, C.M. Comparative study of the neural differentiation capacity of mesenchymal stromal cells from different tissue sources: An approach for their use in neural regeneration therapies. PLoS ONE 2019, 14, e0213032. [Google Scholar] [CrossRef]
- Li, C.; Zhang, M.; Liu, S.Y.; Zhang, F.S.; Wan, T.; Ding, Z.T.; Zhang, P.X. Chitin Nerve Conduits with Three-Dimensional Spheroids of Mesenchymal Stem Cells from SD Rats Promote Peripheral Nerve Regeneration. Polymers 2021, 13, 3957. [Google Scholar] [CrossRef]
- Raoofi, A.; Sadeghi, Y.; Piryaei, A.; Sajadi, E.; Aliaghaei, A.; Rashidiani-Rashidabadi, A.; Fatabadi, F.F.; Mahdavi, B.; Abdollahifar, M.A.; Khaneghah, A.M. Bone Marrow Mesenchymal Stem Cell Condition Medium Loaded on PCL Nanofibrous Scaffold Promoted Nerve Regeneration After Sciatic Nerve Transection in Male Rats. Neurotox. Res. 2021, 39, 1470–1486. [Google Scholar] [CrossRef]
- Tanaka, H.; Kakinoki, R.; Kaizawa, Y.; Yurie, H.; Ikeguchi, R.; Akagi, M. Bone marrow-derived mesenchymal stem cells transplanted into a vascularized biodegradable tube containing decellularized allogenic nerve basal laminae promoted peripheral nerve regeneration; can it be an alternative of autologous nerve graft? PLoS ONE 2021, 16, e0254968. [Google Scholar] [CrossRef]
- Zhang, R.C.; Du, W.Q.; Zhang, J.Y.; Yu, S.X.; Lu, F.Z.; Ding, H.M.; Cheng, Y.B.; Ren, C.; Geng, D.Q. Mesenchymal stem cell treatment for peripheral nerve injury: A narrative review. Neural. Regen. Res. 2021, 16, 2170–2176. [Google Scholar] [CrossRef]
- Hernández, R.; Jiménez-Luna, C.; Perales-Adán, J.; Perazzoli, G.; Melguizo, C.; Prados, J. Differentiation of human mesenchymal stem cells towards neuronal lineage: Clinical trials in nervous system disorders. Biomol. Ther. 2020, 28, 34. [Google Scholar] [CrossRef]
- Scuteri, A.; Miloso, M.; Foudah, D.; Orciani, M.; Cavaletti, G.; Tredici, G. Mesenchymal stem cells neuronal differentiation ability: A real perspective for nervous system repair? Curr. Stem Cell Res. Ther. 2011, 6, 82–92. [Google Scholar] [CrossRef]
- Cho, Y.M.; Jang, Y.-S.; Jang, Y.-M.; Chung, S.-M.; Kim, H.-S.; Lee, J.-H.; Jeong, S.-W.; Kim, I.-K.; Kim, J.J.; Kim, K.-S. Induction of unfolded protein response during neuronal induction of rat bone marrow stromal cells and mouse embryonic stem cells. Exp. Mol. Med. 2009, 41, 440–452. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.G.; Ohn, T.; Jang, C.H.; Vijayakumar, K.; Cho, G.W. The Role of Stress Granules in the Neuronal Differentiation of Stem Cells. Mol. Cells 2020, 43, 848–855. [Google Scholar] [CrossRef]
- Abdullah, R.H.; Yaseen, N.Y.; Salih, S.M.; Al-Juboory, A.A.; Hassan, A.; Al-Shammari, A.M. Induction of mice adult bone marrow mesenchymal stem cells into functional motor neuron-like cells. J. Chem. Neuroanat. 2016, 77, 129–142. [Google Scholar] [CrossRef]
- Zheng, K.; Feng, G.; Zhang, J.; Xing, J.; Huang, D.; Lian, M.; Zhang, W.; Wu, W.; Hu, Y.; Lu, X.; et al. Basic fibroblast growth factor promotes human dental pulp stem cells cultured in 3D porous chitosan scaffolds to neural differentiation. Int. J. Neurosci. 2021, 131, 625–633. [Google Scholar] [CrossRef]
- Farhang, S.; Soleimani, M.; Ostadsharif, M.; Ghasemi, N. Neurogenic induction of human dental pulp derived stem cells by hanging drop technique, basic fibroblast growth factor, and SHH factors. Dent. Res. J. 2021, 18, 57. [Google Scholar]
- Hu, F.; Wang, X.; Liang, G.; Lv, L.; Zhu, Y.; Sun, B.; Xiao, Z. Effects of epidermal growth factor and basic fibroblast growth factor on the proliferation and osteogenic and neural differentiation of adipose-derived stem cells. Cell. Reprogram. 2013, 15, 224–232. [Google Scholar] [CrossRef]
- Qian, Y.; Zhou, X.; Zhang, F.; Diekwisch, T.G.; Luan, X.; Yang, J. Triple PLGA/PCL scaffold modification including silver impregnation, collagen coating, and electrospinning significantly improve biocompatibility, antimicrobial, and osteogenic properties for orofacial tissue regeneration. ACS Appl. Mater. Interfaces 2019, 11, 37381–37396. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.; Milthorpe, B.K.; Herbert, B.R.; Padula, M.P. Proteomic Analysis of Human Adipose Derived Stem Cells during Small Molecule Chemical Stimulated Pre-neuronal Differentiation. Int. J. Stem Cells 2017, 10, 193–217. [Google Scholar] [CrossRef] [Green Version]
- Joe, I.S.; Jeong, S.G.; Cho, G.W. Resveratrol-induced SIRT1 activation promotes neuronal differentiation of human bone marrow mesenchymal stem cells. Neurosci. Lett. 2015, 584, 97–102. [Google Scholar] [CrossRef]
- Hussin, H.M.; Lawi, M.M.; Haflah, N.H.M.; Kassim, A.Y.M.; Idrus, R.B.H.; Lokanathan, Y. Centella asiatica (L.)-Neurodifferentiated Mesenchymal Stem Cells Promote the Regeneration of Peripheral Nerve. Tissue Eng. Regen. Med. 2020, 17, 237–251. [Google Scholar] [CrossRef]
- Petrova, E.S.; Kolos, E.A.; Korzhevskii, D.E. Changes in the Thickness of Rat Nerve Sheaths after Single Subperineural Administration of Rat Bone Marrow Mesenchymal Stem Cells. Bull. Exp. Biol. Med. 2021, 171, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef] [Green Version]
- Jessen, K.R.; Mirsky, R.; Lloyd, A.C. Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harb. Perspect. Biol. 2015, 7, a020487. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Belfiore, L.; Chu, T.H.; Fleming, T.; Midha, R.; Biernaskie, J.; Schuurmans, C. Insights Into the Role and Potential of Schwann Cells for Peripheral Nerve Repair From Studies of Development and Injury. Front. Mol. Neurosci. 2020, 13, 608442. [Google Scholar] [CrossRef]
- Matsuse, D.; Kitada, M.; Kohama, M.; Nishikawa, K.; Makinoshima, H.; Wakao, S.; Fujiyoshi, Y.; Heike, T.; Nakahata, T.; Akutsu, H.; et al. Human umbilical cord-derived mesenchymal stromal cells differentiate into functional Schwann cells that sustain peripheral nerve regeneration. J. Neuropathol. Exp. Neurol. 2010, 69, 973–985. [Google Scholar] [CrossRef] [Green Version]
- Zaminy, A.; Shokrgozar, M.A.; Sadeghi, Y.; Noroozian, M.; Heidari, M.H.; Piryaei, A. Mesenchymal stem cells as an alternative for Schwann cells in rat spinal cord injury. Iran. Biomed. J. 2013, 17, 113–122. [Google Scholar] [CrossRef]
- Alizadeh-Mohajer, M.; Raisi, A.; Farjanikish, G.; Mohammadi, R. Effect of Local Administration of Verapamil Combined with Chitosan Based Hybrid Nanofiber Conduit on Transected Sciatic Nerve in Rat. Bull. Emerg. Trauma 2019, 7, 28–34. [Google Scholar] [CrossRef]
- Dębski, T.; Kijeńska-Gawrońska, E.; Zołocińska, A.; Siennicka, K.; Słysz, A.; Paskal, W.; Włodarski, P.K.; Święszkowski, W.; Pojda, Z. Bioactive Nanofiber-Based Conduits in a Peripheral Nerve Gap Management-An Animal Model Study. Int. J. Mol. Sci. 2021, 22, 5588. [Google Scholar] [CrossRef]
- Jahromi, M.; Razavi, S.; Seyedebrahimi, R.; Reisi, P.; Kazemi, M. Regeneration of Rat Sciatic Nerve Using PLGA Conduit Containing Rat ADSCs with Controlled Release of BDNF and Gold Nanoparticles. J. Mol. Neurosci. 2021, 71, 746–760. [Google Scholar] [CrossRef]
- Rao, F.; Wang, Y.; Zhang, D.; Lu, C.; Cao, Z.; Sui, J.; Wu, M.; Zhang, Y.; Pi, W.; Wang, B.; et al. Aligned chitosan nanofiber hydrogel grafted with peptides mimicking bioactive brain-derived neurotrophic factor and vascular endothelial growth factor repair long-distance sciatic nerve defects in rats. Theranostics 2020, 10, 1590–1603. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.H.; Lee, S.; Park, I.Y.; Song, A.; Moon, C.; Cho, G.W. Memantine Attenuates Salicylate-induced Tinnitus Possibly by Reducing NR2B Expression in Auditory Cortex of Rat. Exp. Neurobiol. 2019, 28, 495–503. [Google Scholar] [CrossRef] [PubMed]













| Gene | Forward Primer (5′ → 3′) | Reverse Primer (5′ → 3′) |
|---|---|---|
| CD133 | GAGCAGGTTGTGTGCTTGGT | GGAAGCACTGGATCTGCTGAA |
| GFAP | CAGAAGAGGACACAATGGCG | GTACAGAGCAAGAAGGGCTG |
| Musashi | TCTGTGTAGGGGGACTGTGT | TGAATGGCACAGACCAGGAA |
| Nestin | GATAAGTCAGCCAGGGAGCAG | GACATCTTGAGGTGCGCCAG |
| ANG | TGGGCGTTTTGTTGTTGGTC | GGCATCATAGTGCTGGGTCA |
| BDNF | ACCCACACGCTTCTGTATGG | GCAGCCTTCATGCAACCAAA |
| bFGF | AAAAACGGGGGCTTCTTCCT | ACGGTTAGCACACACTCCTT |
| VEGF | AGAAAATCCCTGTGGGCCTT | GTCACATCTGCAAGTACGTTCG |
| p21 | GTCTTGTACCCTTGTGCCTC | GGCGTTTGGAGTGGTAGAAA |
| β-actin | ATCCGCAAAGACCTGTACGC | TCTTCATTGTGCTGGGTGCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, G.; Moon, C.; Maharajan, N.; Ang, M.J.; Kim, M.; Jang, C.H. Effect of Pre-Induced Mesenchymal Stem Cell-Coated Cellulose/Collagen Nanofibrous Nerve Conduit on Regeneration of Transected Facial Nerve. Int. J. Mol. Sci. 2022, 23, 7638. https://doi.org/10.3390/ijms23147638
Cho G, Moon C, Maharajan N, Ang MJ, Kim M, Jang CH. Effect of Pre-Induced Mesenchymal Stem Cell-Coated Cellulose/Collagen Nanofibrous Nerve Conduit on Regeneration of Transected Facial Nerve. International Journal of Molecular Sciences. 2022; 23(14):7638. https://doi.org/10.3390/ijms23147638
Chicago/Turabian StyleCho, GwangWon, Changjong Moon, Nagarajan Maharajan, Mary Jasmin Ang, Minseong Kim, and Chul Ho Jang. 2022. "Effect of Pre-Induced Mesenchymal Stem Cell-Coated Cellulose/Collagen Nanofibrous Nerve Conduit on Regeneration of Transected Facial Nerve" International Journal of Molecular Sciences 23, no. 14: 7638. https://doi.org/10.3390/ijms23147638