The Effect of Pore Size Distribution and l-Lysine Modified Apatite Whiskers (HAP) on Osteoblasts Response in PLLA/HAP Foam Scaffolds Obtained in the Thermally Induced Phase Separation Process
Abstract
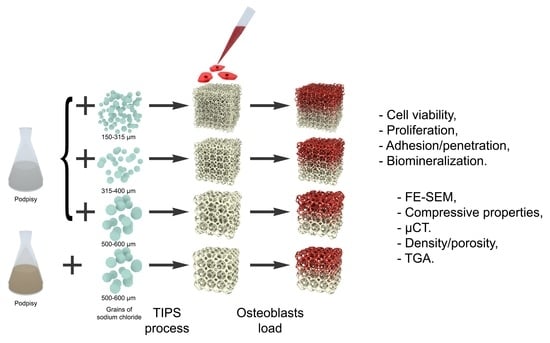
:1. Introduction
2. Results and Discussion
2.1. Apatite Properties
2.2. PLLA/HAP Scaffolds Physical Properties
2.3. Biological Evaluation of Osteoblasts Exposed to the PLLA/HAP Scaffolds Viability of hFOB 1.19 Osteoblasts
2.3.1. hFOB 1.19 Proliferation
2.3.2. Cell Attachment and Penetration
2.3.3. The Levels of Calcium Deposited on the PLLA/HAP Scaffolds
3. Materials and Methods
3.1. Materials
3.2. Apatite Whiskers (HAP) Synthesis and Modification with l-Lysine
3.3. PLLA Foam Scaffolds Preparation
3.4. Scanning Electron Microscopy
3.5. Compressive Strength
3.6. Computer Tomography Analysis (CT)
3.7. Porosity Measurements
3.8. Thermogravimetry (TGA)
3.9. Bioefficacy of the PLLA/HAP Foam Scaffolds
3.9.1. Sterilization
3.9.2. Cell Culture and Propagation
3.9.3. Direct Contact Cytotoxicity Assay
3.9.4. Cell Proliferation Assay
3.9.5. Visualization of Cell Adhesion and Penetration
3.9.6. SEM and Calcium Deposit Quantification
3.9.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ignjatović, N.; Savić, V.; Najman, S.; Plavšić, M.; Uskoković, D. A study of HAp/PLLA composite as a substitute for bone powder, using FT-IR spectroscopy. Biomaterials 2001, 22, 571–575. [Google Scholar] [CrossRef]
- Nishida, Y.; Domura, R.; Sakai, R.; Okamoto, M.; Arakawa, S.; Ishiki, R.; Salick, M.R.; Turng, L.S. Fabrication of PLLA/HA composite scaffolds modified by DNA. Polymer 2015, 56, 73–81. [Google Scholar] [CrossRef]
- Wang, X.; Song, G.; Lou, T. Fabrication and characterization of nano-composite scaffold of PLLA/silane modified hydroxyapatite. Med. Eng. Phys. 2010, 32, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Davachi, S.M.; Kaffashi, B.; Torabinejad, B.; Zamanian, A.; Seyfi, J.; Hejazi, I. Investigating thermal, mechanical and rheological properties of novel antibacterial hybrid nanocomposites based on PLLA/triclosan/nano- hydroxyapatite. Polymer 2016, 90, 232–241. [Google Scholar] [CrossRef]
- Mansourizadeh, F.; Asadi, A.; Oryan, S.; Nematollahzadeh, A.; Dodel, M.; Asghari-Vostakolaei, M. PLLA/HA Nano composite scaffolds for stem cell proliferation and differentiation in tissue engineering. Mol. Biol. Res. Commun. 2013, 2, 1–10. [Google Scholar]
- Szustakiewicz, K.; Kryszak, B.; Gazińska, M.; Chęcmanowski, J.; Stępak, B.; Grzymajło, M.; Antończak, A. The effect of selective mineralization of PLLA in simulated body fluid induced by ArF excimer laser irradiation: Tailored composites with potential in bone tissue engineering. Compos. Sci. Technol. 2020, 197, 108279. [Google Scholar] [CrossRef]
- Liu, K.; Li, W.; Chen, S.; Wen, W.; Lu, L.; Liu, M.; Zhou, C.; Luo, B. The design, fabrication and evaluation of 3D printed gHNTs/gMgO whiskers/PLLA composite scaffold with honeycomb microstructure for bone tissue engineering. Compos. Part B Eng. 2020, 192, 108001. [Google Scholar] [CrossRef]
- Anderson, J.M.; Shive, M.S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 2012, 64, 72–82. [Google Scholar] [CrossRef]
- Guo, J.; Su, W.; Jiang, J.; Ning, C.; Zhao, J.; Liu, X. Enhanced tendon to bone healing in rotator cuff tear by PLLA/CPS composite films prepared by a simple melt-pressing method: An in vitro and in vivo study. Compos. Part B Eng. 2019, 165, 526–536. [Google Scholar] [CrossRef]
- Ignjatović, N.; Tomić, S.; Dakić, M.; Miljković, M.; Plavšić, M.; Uskoković, D. Synthesis and properties of hydroxyapatite/poly-l-lactide composite biomaterials. Biomaterials 1999, 20, 809–816. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, H.; Geng, H.; Liu, D.; Zhang, X.; Cai, Q.; Yang, X. Dual functional polylactide–hydroxyapatite nanocomposites for bone regeneration with nano- silver being loaded via reductive polydopamine. RSC Adv. 2016, 6, 91349–91360. [Google Scholar] [CrossRef]
- Wei, G.; Ma, P.X. Macroporous and nanofibrous polymer scaffolds and polymer/bone-like apatite composite scaffolds generated by sugar spheres. J. Biomed. Mater. Res. Part A 2006, 78, 306–315. [Google Scholar] [CrossRef] [Green Version]
- Szustakiewicz, K.; Stępak, B.; Antończak, A.J.; Maj, M.; Gazińska, M.; Kryszak, B.; Pigłowski, J. Femtosecond laser-induced modification of PLLA/hydroxypatite composite. Polym. Degrad. Stab. 2018, 149, 152–161. [Google Scholar] [CrossRef]
- Zhang, H.; Darvell, B.W. Synthesis and characterization of hydroxyapatite whiskers by hydrothermal homogeneous precipitation using acetamide. Acta Biomater. 2010, 6, 3216–3222. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, S.C.; Lieblich, M.; López, F.A.; Benavente, R.; González-Carrasco, J.L. Effect of Mg content on the thermal stability and mechanical behaviour of PLLA/Mg composites processed by hot extrusion. Mater. Sci. Eng. C 2017, 72, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Gazińska, M.; Krokos, A.; Kobielarz, M.; Włodarczyk, M.; Skibińska, P.; Stępak, B.; Antończak, A.; Morawiak, M.; Płociński, P.; Rudnicka, K. Influence of hydroxyapatite surface functionalization on thermal and biological properties of poly(l-lactide)-and poly(l-lactide-co-glycolide)-based composites. Int. J. Mol. Sci. 2020, 21, 6711. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Tang, D.; Lu, Z.; Xin, Z.; Song, J.; Meng, J.; Lu, J.R.; Li, Z.; Li, J. Ultrafast bone-like apatite formation on highly porous poly(l-lactic acid)-hydroxyapatite fibres. Mater. Sci. Eng. C 2020, 116, 111168. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Pyda, M.; Cebe, P. Electrospun fibers of poly(l-lactic acid) containing lovastatin with potential applications in drug delivery. J. Appl. Polym. Sci. 2017, 134, 45287. [Google Scholar] [CrossRef]
- Szustakiewicz, K.; Gazińska, M.; Kryszak, B.; Grzymajło, M.; Pigłowski, J.; Wiglusz, R.J.; Okamoto, M. The influence of hydroxyapatite content on properties of poly(l-lactide)/hydroxyapatite porous scaffolds obtained using thermal induced phase separation technique. Eur. Polym. J. 2019, 113, 313–320. [Google Scholar] [CrossRef]
- Schugens, C.; Maquet, V.; Grandfils, C.; Jerome, R.; Teyssie, P. Biodegradable and macropororous polylactide implants for cell transplantation: 1. Preparation of macroporus polylactide supports by solid-liquid phase separation. Polymer 1996, 37, 1027–1038. [Google Scholar] [CrossRef] [Green Version]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.J.; Kasper, F.K.; Mikos, A.G. Biomaterials for tissue engineering. Ann. Biomed. Eng. 2014, 42, 323–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, T.; Wang, X.; Yan, X.; Miao, Y.; Long, Y.-Z.; Yin, H.-L.; Sun, B.; Song, G. Fabrication and biocompatibility of poly (l-lactic acid) and chitosan composite scaffolds with hierarchical microstructures. Mater. Sci. Eng. C 2016, 64, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Sencadas, V.; Sadat, S.; Silva, D.M. Mechanical performance of elastomeric PGS scaffolds under dynamic conditions. J. Mech. Behav. Biomed. Mater. 2020, 102. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, P.; Cannillo, V.; Sola, A.; Dorigato, A.; Chiellini, F. Highly porous polycaprolactone-45S5 Bioglass® scaffolds for bone tissue engineering. Compos. Sci. Technol. 2010, 70, 1869–1878. [Google Scholar] [CrossRef] [Green Version]
- Papenburg, B.J.; Liu, J.; Higuera, G.A.; Barradas, A.M.C.; de Boer, J.; van Blitterswijk, C.A.; Wessling, M.; Stamatialis, D. Development and analysis of multi-layer scaffolds for tissue engineering. Biomaterials 2009, 30, 6228–6239. [Google Scholar] [CrossRef]
- Fini, M.; Torricelli, P.; Giavaresi, G.; Carpi, A.; Nicolini, A.; Giardino, R. Effect of l-lysine and l-arginine on primary osteoblast cultures from normal and osteopenic rats. Biomed. Pharmacother. 2001, 55, 213–220. [Google Scholar] [CrossRef]
- Torricelli, P.; Fini, M.; Giavaresi, G.; Giardino, R.; Gnudi, S.; Nicolini, A.; Carpi, A. l-Arginine and l-Lysine stimulation on cultured human osteoblasts. Biomed. Pharmacother. 2002, 56, 492–497. [Google Scholar] [CrossRef]
- Liuyun, J.; Lixin, J.; Chengdong, X.; Lijuan, X.; Ye, L. Effect of l-lysine-assisted surface grafting for nano-hydroxyapatite on mechanical properties and in vitro bioactivity of poly(lactic acid-co-glycolic acid). J. Biomater. Appl. 2016, 30, 750–758. [Google Scholar] [CrossRef]
- Zheng, S.; Guan, Y.; Yu, H.; Huang, G.; Zheng, C. Poly-l-lysine-coated PLGA/poly(amino acid)-modified hydroxyapatite porous scaffolds as efficient tissue engineering scaffolds for cell adhesion, proliferation, and differentiation. New J. Chem. 2019, 43, 9989–10002. [Google Scholar] [CrossRef]
- Biernat, M.; Jaegermann, Z.; Tymowicz-Grzyb, P.; Konopka, G. Influence of low-temperature reaction time on morphology and phase composition of short calcium phosphate whiskers. Process. Appl. Ceram. 2019, 13, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Martz, E.O.; Goel, V.K.; Pope, M.H.; Park, J.B. Materials and design of spinal implants—A review. J. Biomed. Mater. Res. 1997, 38, 267–288. [Google Scholar] [CrossRef]
- Klein, C.P.A.T.; Driessen, A.A.; de Groot, K.; van den Hooff, A. Biodegradation behavior of various calcium phosphate materials in bone tissue. J. Biomed. Mater. Res. 1983, 17, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Chang, B.S.; Jeung, U.O.; Park, K.W.; Kim, M.S.; Lee, C.K. The first clinical trial of beta-calcium pyrophosphate as a novel bone graft extender in instrumented posterolateral Lumbar fusion. Clin. Orthop. Surg. 2011, 3, 238–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.H.; Loo, C.Y.; Zavgorodniy, A.V.; Rohanizadeh, R. High protein adsorptive capacity of amino acid-functionalized hydroxyapatite. J. Biomed. Mater. Res. Part A 2013, 101 A, 873–883. [Google Scholar] [CrossRef]
- Ozhukil Kollath, V.; Van Den Broeck, F.; Fehér, K.; Martins, J.C.; Luyten, J.; Traina, K.; Mullens, S.; Cloots, R. A Modular Approach to Study Protein Adsorption on Surface Modified Hydroxyapatite. Chem. Eur. J. 2015, 21, 10497–10505. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Salick, M.R.; Cordie, T.; Crone, W.C.; Peng, X.-F.; Turng, L.-S. Morphology, mechanical properties, and shape memory effects of poly(lactic acid)/ thermoplastic polyurethane blend scaffolds prepared by thermally induced phase separation. J. Cell. Plast. 2014, 50, 361–379. [Google Scholar] [CrossRef]
- Önder, Ö.C.; Yilgör, E.; Yilgör, I. Fabrication of rigid poly(lactic acid) foams via thermally induced phase separation. Polymer 2016, 107, 240–248. [Google Scholar] [CrossRef]
- Chen, J.S.; Tu, S.L.; Tsay, R.Y. A morphological study of porous polylactide scaffolds prepared by thermally induced phase separation. J. Taiwan Inst. Chem. Eng. 2010, 41, 229–238. [Google Scholar] [CrossRef]
- Liu, C.G.; Zeng, Y.T.; Kankala, R.K.; Zhang, S.S.; Chen, A.Z.; Wang, S. Bin Characterization and preliminary biological evaluation of 3D-printed porous scaffolds for engineering bone tissues. Materials 2018, 11, 1832. [Google Scholar] [CrossRef] [Green Version]
- Venugopal, J.; Low, S.; Choon, A.T.; Sampath Kumar, T.S.; Ramakrishna, S. Mineralization of osteoblasts with electrospun collagen/hydroxyapatite nanofibers. J. Mater. Sci. Mater. Med. 2008, 19, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite—Past, Present, and Future in Bone Regeneration. Bone Tissue Regen. Insights 2016, 7, BTRI.S36138. [Google Scholar] [CrossRef] [Green Version]
- González Ocampo, J.I.; Bassous, N.; Ossa Orozco, C.P.; Webster, T.J. Evaluation of cytotoxicity and antimicrobial activity of an injectable bone substitute of carrageenan and nano hydroxyapatite. J. Biomed. Mater. Res. Part A 2018, 106, 2984–2993. [Google Scholar] [CrossRef] [PubMed]
- Tas, A.C. Formation of calcium phosphate whiskers in hydrogen peroxide (H2O2) solutions at 90 °C. J. Am. Ceram. Soc. 2007, 90, 2358–2362. [Google Scholar] [CrossRef] [Green Version]
- Feldkamp, L.A.; Davis, L.C.; Kress, J.W. Practical cone-beam algorithm. J. Opt. Soc. Am. A 1984, 1, 612–619. [Google Scholar] [CrossRef] [Green Version]







| Sample | Density ρsc (g·cm−3) | Porosity Φp (%) |
|---|---|---|
| PLLA/HAP_150–315 | 0.037±0.005 | 98.0±0.1 |
| PLLA/HAP_315–400 | 0.030±0.002 | 98.4±0.1 |
| PLLA/HAP_500–600 | 0.032±0.005 | 98.2±0.3 |
| PLLA/HAP_500–600_lys | 0.036±0.004 | 98.5±0.2 |
| Sample | Compressive Modulus (kPa) | Compressive Stress (40%strain) (kPa) | Compressive Stress (80%strain) (kPa) |
|---|---|---|---|
| PLLA/HAP_150–315 | 185±23 | 24.3±2.5 | 190±17.9 |
| PLLA/HAP_315–400 | 160±11 | 20.2±1.2 | 147.4±7.8 |
| PLLA/HAP_500–600 | 169±23 | 29.6±3.0 | 169.3±23.9 |
| PLLA/HAP_500–600_lys | 250±25 | 26.5±3.9 | 173.8±20.3 |
| Sample Designation | Apatite Content (wt.%) | Size of Sodium Chloride Used for the Salt Leaching Procedure (μm) |
|---|---|---|
| PLLA/HAP 150–315 | 50 | 150–315 |
| PLLA/HAP 315–400 | 50 | 315–400 |
| PLLA/HAP 500–600 | 50 | 500–600 |
| PLLA/HAP 500–600_lys | 50 | 500–600 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szustakiewicz, K.; Włodarczyk, M.; Gazińska, M.; Rudnicka, K.; Płociński, P.; Szymczyk-Ziółkowska, P.; Ziółkowski, G.; Biernat, M.; Sieja, K.; Grzymajło, M.; et al. The Effect of Pore Size Distribution and l-Lysine Modified Apatite Whiskers (HAP) on Osteoblasts Response in PLLA/HAP Foam Scaffolds Obtained in the Thermally Induced Phase Separation Process. Int. J. Mol. Sci. 2021, 22, 3607. https://doi.org/10.3390/ijms22073607
Szustakiewicz K, Włodarczyk M, Gazińska M, Rudnicka K, Płociński P, Szymczyk-Ziółkowska P, Ziółkowski G, Biernat M, Sieja K, Grzymajło M, et al. The Effect of Pore Size Distribution and l-Lysine Modified Apatite Whiskers (HAP) on Osteoblasts Response in PLLA/HAP Foam Scaffolds Obtained in the Thermally Induced Phase Separation Process. International Journal of Molecular Sciences. 2021; 22(7):3607. https://doi.org/10.3390/ijms22073607
Chicago/Turabian StyleSzustakiewicz, Konrad, Marcin Włodarczyk, Małgorzata Gazińska, Karolina Rudnicka, Przemysław Płociński, Patrycja Szymczyk-Ziółkowska, Grzegorz Ziółkowski, Monika Biernat, Katarzyna Sieja, Michał Grzymajło, and et al. 2021. "The Effect of Pore Size Distribution and l-Lysine Modified Apatite Whiskers (HAP) on Osteoblasts Response in PLLA/HAP Foam Scaffolds Obtained in the Thermally Induced Phase Separation Process" International Journal of Molecular Sciences 22, no. 7: 3607. https://doi.org/10.3390/ijms22073607