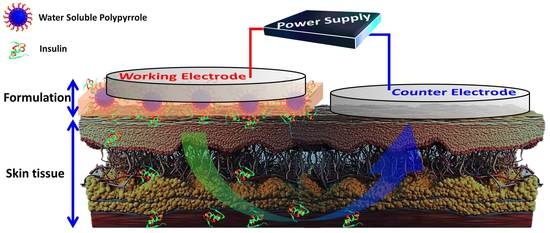
Controlled Transdermal Iontophoresis of Insulin from Water-Soluble Polypyrrole Nanoparticles: An In Vitro Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Obtained WS-PPyNPs
2.2. Stability of INS
2.3. The Loading Mechanism
2.4. Optimization of Experimental Conditions
2.4.1. Effect of Current Density
2.4.2. Effect of pH of the Formulation
2.4.3. Effect of INS Concentration
2.4.4. Effect of Sodium Chloride Concentration
2.5. INS Transdermal Delivery
3. Experimental Procedures
3.1. Materials
3.2. Synthesis of WS-PPyNPs
3.3. INS Loading Experiments
3.4. Characterizations of the Obtained and INS-Loaded Matrix
3.5. Skin Preparation
3.6. In Vitro Permeation Experiments
3.7. Effect of Experimental Conditions on IP
3.8. Stability of INS in the Presence of Electrical Current
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Iyer, H.; Khedkar, A.; Verma, M. Oral insulin–a review of current status. Diabetes Obes. Metab. 2010, 12, 179–185. [Google Scholar] [CrossRef]
- Gurevich-Panigrahi, T.; Panigrahi, S.; Wiechec, E.; Los, M. Obesity: pathophysiology and clinical management. Curr. Med. Chem. 2009, 16, 506–521. [Google Scholar] [CrossRef] [Green Version]
- Federation, I.D. IDF Diabetes Atlas-9th Edition. 2019. Available online: https://www.diabetesatlas.org/en/ (accessed on 20 October 2020).
- Whitley, H.P.; Lee, R.; Steil, C.; Pillion, D. Student pharmacists’ service-oriented learning at a camp for children with type 1 diabetes mellitus. Curr. Pharm. Teach. Learn. 2019, 11, 825–831. [Google Scholar] [CrossRef]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef] [Green Version]
- Owens, D.R.; Zinman, B.; Bolli, G. Alternative routes of insulin delivery. Diabet. Med. 2003, 20, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Jiang, T.; Di, J.; Tai, W.; Gu, Z. Emerging micro-and nanotechnology based synthetic approaches for insulin delivery. Chem. Soc. Rev. 2014, 43, 3595–3629. [Google Scholar] [CrossRef]
- Bakh, N.A.; Cortinas, A.B.; Weiss, M.A.; Langer, R.S.; Anderson, D.G.; Gu, Z.; Dutta, S.; Strano, M.S. Glucose-responsive insulin by molecular and physical design. Nat. Chem. 2017, 9, 937–943. [Google Scholar] [CrossRef]
- Martanto, W.; Davis, S.P.; Holiday, N.R.; Wang, J.; Gill, H.S.; Prausnitz, M.R. Transdermal delivery of insulin using microneedles in vivo. Pharm. Res. 2004, 21, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Song, C.; Baik, S.; Kim, D.; Hyeon, T.; Kim, D.-H. Device-assisted transdermal drug delivery. Adv. Drug Deliv. Rev. 2018, 127, 35–45. [Google Scholar] [CrossRef]
- Pikal, M.J. The role of electroosmotic flow in transdermal iontophoresis. Adv. Drug Deliv. Rev. 2001, 46, 281–305. [Google Scholar] [CrossRef]
- Matos, B.N.; Pereira, M.N.; Bravo, M.d.O.; Cunha-Filho, M.; Saldanha-Araújo, F.; Gratieri, T.; Gelfuso, G.M. Chitosan nanoparticles loading oxaliplatin as a mucoadhesive topical treatment of oral tumors: Iontophoresis further enhances drug delivery ex vivo. Int. J. Biol. Macromol. 2020, 154, 1265–1275. [Google Scholar] [CrossRef]
- Bernardi, D.S.; Bitencourt, C.; da Silveira, D.S.C.; da Cruz, E.L.C.M.; Pereira-da-Silva, M.A.; Faccioli, L.H.; Lopez, R.F.V. Effective transcutaneous immunization using a combination of iontophoresis and nanoparticles. Nanotechnol. Biol. Med. 2016, 12, 2439–2448. [Google Scholar] [CrossRef]
- Khafagy, E.-S.; Morishita, M.; Onuki, Y.; Takayama, K. Current challenges in non-invasive insulin delivery systems: a comparative review. Adv. Drug Deliv. Rev. 2007, 59, 1521–1546. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Kahkoska, A.R.; Wang, J.; Buse, J.B.; Gu, Z. Advances in transdermal insulin delivery. Adv. Drug Deliv. Rev. 2019, 139, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhu, H.; Zheng, J.; Mou, D.; Wan, J.; Zhang, J.; Shi, T.; Zhao, Y.; Xu, H.; Yang, X. Iontophoresis-driven penetration of nanovesicles through microneedle-induced skin microchannels for enhancing transdermal delivery of insulin. J. Control. Release 2009, 139, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Pillai, O.; Panchagnula, R. Transdermal delivery of insulin from poloxamer gel: Ex vivo and in vivo skin permeation studies in rat using iontophoresis and chemical enhancers. J. Control. Release 2003, 89, 127–140. [Google Scholar] [CrossRef]
- Rastogi, R.; Anand, S.; Dinda, A.K.; Koul, V. Investigation on the synergistic effect of a combination of chemical enhancers and modulated iontophoresis for transdermal delivery of insulin. Drug Dev. Ind. Pharm. 2010, 36, 993–1004. [Google Scholar] [CrossRef]
- Pillai, O.; Nair, V.; Panchagnula, R. Transdermal iontophoresis of insulin: IV. Influence of chemical enhancers. Int. J. Pharm. 2004, 269, 109–120. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Madrakian, T.; Ghoorchian, A.; Kamalabadi, M.; Afkhami, A. Stimuli-sensitive drug delivery systems. In Nanoengineered Biomaterials for Advanced Drug Delivery; Mozafari, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 37–59. [Google Scholar]
- Zhu, C.; Liu, L.; Yang, Q.; Lv, F.; Wang, S. Water-soluble conjugated polymers for imaging, diagnosis, and therapy. Chem. Rev. 2012, 112, 4687–4735. [Google Scholar] [CrossRef]
- Cheng, C.C.; Lai, Y.C.; Shieh, Y.T.; Chang, Y.H.; Lee, A.W.; Chen, J.K.; Lee, D.J.; Lai, J.Y. CO2-Responsive Water-Soluble Conjugated Polymers for in Vitro and in Vivo Biological Imaging. Biomacromolecules 2020, 21, 5282–5291. [Google Scholar] [CrossRef]
- Zhou, L.; Lv, F.; Liu, L.; Wang, S. Water-Soluble Conjugated Organic Molecules as Optical and Electrochemical Materials for Interdisciplinary Biological Applications. Acc. Chem. Res. 2019, 52, 3211–3222. [Google Scholar] [CrossRef]
- Antony, M.J.; Jayakannan, M. Amphiphilic Azobenzenesulfonic Acid Anionic Surfactant for Water-Soluble, Ordered, and Luminescent Polypyrrole Nanospheres. J. Phys. Chem. B 2007, 111, 12772–12780. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, N.; Akbarinejad, A.; Ghoorchian, A. Photophysical diversity of water-soluble fluorescent conjugated polymers induced by surfactant stabilizers for rapid and highly selective determination of 2, 4, 6-trinitrotoluene traces. ACS Appl. Mater. Interfaces 2016, 8, 24901–24908. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Bai, H.; Shi, G. Conducting polymer nanomaterials: Electrosynthesis and applications. Chem. Soc. Rev. 2009, 38, 2397–2409. [Google Scholar] [CrossRef]
- Bae, W.J.; Kim, K.H.; Jo, W.H.; Park, Y.H. A water-soluble and self-doped conducting polypyrrole graft copolymer. Macromolecules 2005, 38, 1044–1047. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, B.; Ouyang, X.; Chen, L.; Wu, H. Polymer solar cells employing water-soluble polypyrrole nanoparticles as dopants of PEDOT: PSS with enhanced efficiency and stability. J. Phys. Chem. C 2017, 121, 18378–18384. [Google Scholar] [CrossRef]
- Alizadeh, N.; Akbarinejad, A.; Hosseinkhani, S.; Rabbani, F. Synthesis of highly fluorescent water-soluble polypyrrole for cell imaging and iodide ion sensing. Anal. Chim. Acta 2019, 1084, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Ramanaviciene, A.; Kausaite, A.; Tautkus, S.; Ramanavicius, A. Biocompatibility of polypyrrole particles: an in-vivo study in mice. J. Pharm. Pharmacol. 2007, 59, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, X.; Yuan, C.; Chen, S.; Zhang, P.; Zhang, T.; Yao, J.; Chen, F.; Chen, G. Evaluation of biocompatibility of polypyrrole in vitro and in vivo. J. Biomed. Mater. Res. A 2004, 68, 411–422. [Google Scholar] [CrossRef]
- Cho, G.; Fung, B.M.; Glatzhofer, D.T.; Lee, J.-S.; Shul, Y.-G. Preparation and characterization of polypyrrole-coated nanosized novel ceramics. Langmuir 2001, 17, 456–461. [Google Scholar] [CrossRef]
- Omastová, M.; Trchová, M.; Pionteck, J.; Prokeš, J.; Stejskal, J. Effect of polymerization conditions on the properties of polypyrrole prepared in the presence of sodium bis(2-ethylhexyl) sulfosuccinate. Synth. Met. 2004, 143, 153–161. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Song, W.; Liu, Z. Controllable synthesis of conducting polypyrrole nanostructures. J. Phys. Chem. B 2006, 110, 1158–1165. [Google Scholar] [CrossRef]
- Omastová, M.; Trchová, M.; Kovářová, J.; Stejskal, J. Synthesis and structural study of polypyrroles prepared in the presence of surfactants. Synth. Met. 2003, 138, 447–455. [Google Scholar] [CrossRef]
- Dong, A.; Prestrelski, S.J.; Dean Allison, S.; Carpenter, J.F. Infrared Spectroscopic Studies of Lyophilization- and Temperature-Induced Protein Aggregation. J. Pharm. Sci. 1995, 84, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Ganeshkumar, M.; Ponrasu, T.; Sathishkumar, M.; Suguna, L. Preparation of amphiphilic hollow carbon nanosphere loaded insulin for oral delivery. Colloids Surf. B Biointerfaces 2013, 103, 238–243. [Google Scholar] [CrossRef]
- Elsayed, A.; Al-Remawi, M.; Maghrabi, I.; Hamaidi, M.; Jaber, N. Development of insulin loaded mesoporous silica injectable particles layered by chitosan as a controlled release delivery system. Int. J. Pharm. 2014, 461, 448–458. [Google Scholar] [CrossRef]
- Kartal, F.; Çimen, D.; Bereli, N.; Denizli, A. Molecularly imprinted polymer based quartz crystal microbalance sensor for the clinical detection of insulin. Mater. Sci. Eng. C 2019, 97, 730–737. [Google Scholar] [CrossRef]
- Stabryla, L.M.; Johnston, K.A.; Diemler, N.A.; Cooper, V.S.; Millstone, J.E.; Haig, S.-J.; Gilbertson, L.M. Role of bacterial motility in differential resistance mechanisms of silver nanoparticles and silver ions. Nat. Nanotechnol. 2021, 16, 996–1003. [Google Scholar] [CrossRef]
- Pouroutzidou, G.K.; Liverani, L.; Theocharidou, A.; Tsamesidis, I.; Lazaridou, M.; Christodoulou, E.; Beketova, A.; Pappa, C.; Triantafyllidis, K.S.; Anastasiou, A.D. Synthesis and Characterization of Mesoporous Mg-and Sr-Doped Nanoparticles for Moxifloxacin Drug Delivery in Promising Tissue Engineering Applications. Int. J. Mol. Sci. 2021, 22, 577. [Google Scholar] [CrossRef] [PubMed]
- Di Ianni, T.; Bose, R.J.; Sukumar, U.K.; Bachawal, S.; Wang, H.; Telichko, A.; Herickhoff, C.; Robinson, E.; Baker, S.; Vilches-Moure, J.G. Ultrasound/microbubble-mediated targeted delivery of anticancer microRNA-loaded nanoparticles to deep tissues in pigs. J. Control. Release 2019, 309, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, N.; Akbarinejad, A. Soluble fluorescent polymeric nanoparticles based on pyrrole derivatives: synthesis, characterization and their structure dependent sensing properties. J. Mater. Chem. C 2015, 3, 9910–9920. [Google Scholar] [CrossRef]
- Silva, C.P.; Martínez, J.H.; Martínez, K.D.; Farías, M.E.; Leskow, F.C.; Pérez, O.E. Proposed molecular model for electrostatic interactions between insulin and chitosan. Nano-complexation and activity in cultured cells. Colloids Surf. A: Physicochem. Eng. Asp. 2018, 537, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Hosseini-Nassab, N.; Samanta, D.; Abdolazimi, Y.; Annes, J.P.; Zare, R.N. Electrically controlled release of insulin using polypyrrole nanoparticles. Nanoscale 2017, 9, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, M.; Maurizio, S.; Carpi, A. Skin blood flowmotion response to insulin iontophoresis in normal subjects. Microvasc. Res. 2005, 70, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Pillai, O.; Kumar, N.; Dey, C.S.; Borkute, S.; Nagalingam, S.; Panchagnula, R. Transdermal iotophoresis of insulin. Part 1: A study on the issues associated with the use of platinum electrodes on rat skin. J. Pharm. Pharmacol. 2003, 55, 1505–1513. [Google Scholar] [CrossRef]
- Langkjær, L.; Brange, J.; Grodsky, G.M.; Guy, R.H. Iontophoresis of monomeric insulin analogues in vitro: effects of insulin charge and skin pretreatment. J. Control. Release 1998, 51, 47–56. [Google Scholar] [CrossRef]
- Carpenter, F.H. Relationship of structure to biological activity of insulin as revealed by degradative studies. Am. J. Med. 1966, 40, 750–758. [Google Scholar] [CrossRef]
- Pillai, O.; Borkute, S.D.; Sivaprasad, N.; Panchagnula, R. Transdermal iontophoresis of insulin: II. Physicochemical considerations. Int. J. Pharm. 2003, 254, 271–280. [Google Scholar] [CrossRef]
- Kanikkannan, N.; Singh, J.; Ramarao, P. Transdermal iontophoretic delivery of bovine insulin and monomeric human insulin analogue. J. Control. Release 1999, 59, 99–105. [Google Scholar] [CrossRef]
- Rac, V.; Lević, S.; Balanč, B.; Graells, B.O.; Bijelić, G. PVA Cryogel as model hydrogel for iontophoretic transdermal drug delivery investigations. Comparison with PAA/PVA and PAA/PVP interpenetrating networks. Colloids Surf. B Biointerfaces 2019, 180, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Banga, A.K.; Chien, Y.W. Characterization of in vitro transdermal iontophoretic delivery of insulin. Drug Dev. Ind. Pharm. 1993, 19, 2069–2087. [Google Scholar] [CrossRef]
- Yoshida, N.H.; Roberts, M.S. Prediction of cathodal iontophoretic transport of various anions across excised skin from different vehicles using conductivity measurements. J. Pharm. Pharmacol. 1995, 47, 883–890. [Google Scholar] [CrossRef]
- Gangarosa, L.P.; Park, N.-H.; Wiggins, C.A.; Hill, J.M. Increased penetration of nonelectrolytes into mouse skin during iontophoretic water transport (iontohydrokinesis). J. Pharmacol. Exp. Ther. 1980, 212, 377–381. [Google Scholar] [PubMed]
- Yoshida, N.H.; Roberts, M.S. Role of conductivity in iontophoresis. 2. Anodal iontophoretic transport of phenylethylamine and sodium across excised human skin. J. Pharm. Sci. 1994, 83, 344–350. [Google Scholar] [CrossRef]
- Guy, R.H.; Delgado-Charro, M.B.; Kalia, Y.N. Iontophoretic transport across the skin. Skin Pharmacol. Physiol. 2001, 14, 35–40. [Google Scholar] [CrossRef]
- Jeffrey, P.; Coates, J. An equilibrium ultracentrifuge study of the effect of ionic strength on the self-association of bovine insulin. Biochemistry 1966, 5, 3820–3824. [Google Scholar] [CrossRef]
- Pradhan, R.; Kim, Y.-I.; Chang, S.W.; Kim, J.O. Preparation and evaluation of once-daily sustained-release coated tablets of tolterodine-L-tartrate. Int. J. Pharm. 2014, 460, 205–211. [Google Scholar] [CrossRef]
- Banerjee, A.; Chen, R.; Arafin, S.; Mitragotri, S. Intestinal iontophoresis from mucoadhesive patches: a strategy for oral delivery. J. Control. Release 2019, 297, 71–78. [Google Scholar] [CrossRef]
- Fukuta, T.; Oshima, Y.; Michiue, K.; Tanaka, D.; Kogure, K. Non-invasive delivery of biological macromolecular drugs into the skin by iontophoresis and its application to psoriasis treatment. J. Control. Release 2020, 323, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Kalia, Y.N.; Naik, A.; Garrison, J.; Guy, R.H. Iontophoretic drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 619–658. [Google Scholar] [CrossRef]
- Marro, D.; Guy, R.H.; Delgado-Charro, M.B. Characterization of the iontophoretic permselectivity properties of human and pig skin. J. Control. Release 2001, 70, 213–217. [Google Scholar] [CrossRef]
- Godin, B.; Touitou, E. Transdermal skin delivery: predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Deliv. Rev. 2007, 59, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Ritschel, W.; Starzacher, A.; Sabouni, A.; Hussain, A.; Koch, H. Percutaneous absorption of rosmarinic acid in the rat. Methods Find. Exp. Clin. Pharmacol. 1989, 11, 345–352. [Google Scholar] [PubMed]






| Experiment | Q48h (µg cm−2) | INS Flux (µg cm−2 h−1) | Lag Time (min) | Papp (cm s−1) | EF |
|---|---|---|---|---|---|
| Passive | 17.87 ± 4.69 | 0.36 ± 0.09 | 68 ± 12 | 2.88 (± 0.79) × 10−7 | − |
| AIP | 520.0 ± 136.4 | 9.59 ± 2.65 | 91 ± 13 | 7.68 (± 2.15) × 10−6 | 26.7 ± 10.9 |
| CIP | 834.6 ± 218.9 | 15.67 ± 4.24 | 105 ± 18 | 1.25 (± 0.37) × 10−5 | 43.4 ± 18.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tari, K.; Khamoushian, S.; Madrakian, T.; Afkhami, A.; Łos, M.J.; Ghoorchian, A.; Samarghandi, M.R.; Ghavami, S. Controlled Transdermal Iontophoresis of Insulin from Water-Soluble Polypyrrole Nanoparticles: An In Vitro Study. Int. J. Mol. Sci. 2021, 22, 12479. https://doi.org/10.3390/ijms222212479
Tari K, Khamoushian S, Madrakian T, Afkhami A, Łos MJ, Ghoorchian A, Samarghandi MR, Ghavami S. Controlled Transdermal Iontophoresis of Insulin from Water-Soluble Polypyrrole Nanoparticles: An In Vitro Study. International Journal of Molecular Sciences. 2021; 22(22):12479. https://doi.org/10.3390/ijms222212479
Chicago/Turabian StyleTari, Kamran, Soroush Khamoushian, Tayyebeh Madrakian, Abbas Afkhami, Marek Jan Łos, Arash Ghoorchian, Mohammad Reza Samarghandi, and Saeid Ghavami. 2021. "Controlled Transdermal Iontophoresis of Insulin from Water-Soluble Polypyrrole Nanoparticles: An In Vitro Study" International Journal of Molecular Sciences 22, no. 22: 12479. https://doi.org/10.3390/ijms222212479