Optimization of Chitosan–Alginate Microparticles for Delivery of Mangostins to the Colon Area Using Box–Behnken Experimental Design
Abstract
:1. Introduction
2. Results and Discussion
2.1. Mangostin Content in Mangosteen Extract
2.2. Encapsulation Efficiency and Loading Capacity of Mangostin in Chitosan Microparticles
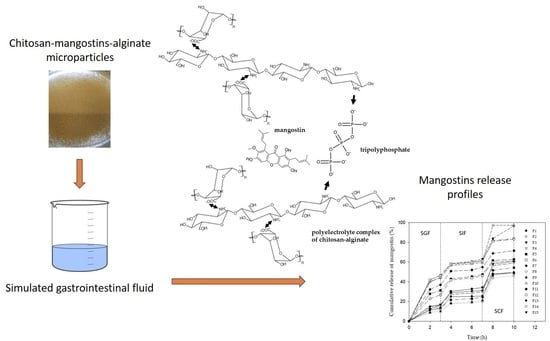
2.3. Cumulative Release of Mangostins from Chitosan-Alginate Microparticles
2.4. Chitosan–Mangostin–Alginate Microparticle Optimization with Box–Behnken Model
2.5. Chitosan–Alginate Microparticles as a pH-Sensitive Delivery Formulation
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Mangosteen Rind Extraction
3.3. Quantification of Mangostins
3.4. Preparation of Chitosan–Mangostin–Alginate Microparticles
3.5. Encapsulation Efficiency and Loading Capacity of Chitosan
3.6. In Vitro Drug Release Study
3.7. Box–Behnken Experimental Design
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Philip, A.K.; Philip, B. Colon targeted drug delivery systems: A review on primary and novel approaches. Oman Med. J. 2010, 25, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Nidhi; Rashid, M.; Kaur, V.; Hallan, S.S.; Sharma, S.; Mishra, N. Microparticles as controlled drug delivery carrier for the treatment of ulcerative colitis. Saudi Pharm. J. 2016, 24, 458–472. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, K.; Akao, Y.; Kobayashi, E.; Ohguchi, K.; Ito, T.; Tanaka, T.; Iinuma, M.; Nozawa, Y. Induction of apoptosis by xanthones from mangosteen in human leukemia cell lines. J. Nat. Prod. 2003, 66, 1124–1127. [Google Scholar] [CrossRef] [PubMed]
- Akao, Y.; Nakagawa, Y.; Iinuma, M.; Nozawa, Y. Anti-cancer effects of xanthones from pericarps of mangosteen. Int. J. Mol. Sci. 2008, 9, 355–370. [Google Scholar] [CrossRef]
- Aisha, A.F.A.; Abu-Salah, K.M.; Ismail, Z.; Majid, A.M.S.A. Quantification of α-, β- and γ-mangostin in Garcinia mangostana fruit rind extracts by a reverse phase high performance liquid chromatography. J. Med. Plant Res. 2012, 6, 4526–4534. [Google Scholar] [CrossRef]
- Aisha, A.F.; Abu-Salah, K.M.; Ismail, Z.; Majid, A. Determination of total xanthones in Garcinia mangostana fruit rind extracts by ultraviolet (UV) spectrophotometry. J. Med. Plant Res. 2013, 7, 29–35. [Google Scholar]
- Pedraza-Chaverri, J.; Cárdenas-Rodríguez, N.; Orozco-Ibarra, M.; Pérez-Rojas, J. Medicinal Properties of Mangosteen (Garcinia mangostana). Food Chem. Toxicol. 2008, 46, 3227–3239. [Google Scholar] [CrossRef]
- Shan, T.; Ma, Q.; Guo, K.; Liu, J.; Li, W.; Wang, F.; Wu, E. Xanthones from Mangosteen Extracts as Natural Chemopreventive Agents: Potential Anticancer Drugs. Curr. Mol. Med. 2011, 11, 666–677. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-based nanomaterials for drug delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [Green Version]
- Krisanti, E.; Aryani, S.D.; Mulia, K. Effect of chitosan molecular weight and composition on mucoadhesive properties of mangostin-loaded chitosan-alginate microparticles. Aip Conf. Proc. 2017, 1817, 020014. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.M.; Paul, W.; Sharma, C.P.; Kuriachan, M.A. Bioadhesive, pH Responsive Micromatrix for Oral Delivery of Insulin. Biomat. Artif. Organs 2005, 18, 198–202. [Google Scholar]
- Elsaid Ali, A.; Taher, M.; Mohamed, F. Microencapsulation of Alpha-Mangostin into PLGA Microspheres and Optimization Using Response Surface Methodology Intended for Pulmonary Delivery. J. Microencapsul. 2013, 30, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Zeng, Z.W.; Xiao, R.Z.; Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar] [CrossRef] [Green Version]
- Sinha, V.; Singla, A.; Wadhawan, S.; Kaushik, R.; Kumria, R.; Bansal, K.; Dhawan, S. Chitosan Microspheres as A Potential Carrier for Drugs. Int. J. Pharm. 2004, 274, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, R.; Amiji, M. Chitosan-Based Gastrointestinal Delivery Systems. J. Control. Release 2003, 89, 151–165. [Google Scholar] [CrossRef]
- Suksamran, T.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. Development of Alginate/Chitosan Microparticles for Dust Mite Allergen. Trop. J. Pharm. Res. 2011, 10, 3. [Google Scholar] [CrossRef] [Green Version]
- He, P.; Davis, S.; Illum, L. In Vitro Evaluation of The Mucoadhesive Properties of Chitosan Microspheres. Int. J. Pharm. 1998, 166, 75–88. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohamadi, N.; Zarrabi, A.; Abasi, S.; Dehghannoudeh, G.; Tamaddondoust, R.N.; Khanbabaei, H.; Mohammadinejad, R.; Thakur, V.K. Chitosan-based advanced materials for docetaxel and paclitaxel delivery: Recent advances and future directions in cancer theranostics. Int. J. Biol. Macromol. 2020, 145, 282–300. [Google Scholar] [CrossRef]
- Shakeri, S.; Ashrafizadeh, M.; Zarrabi, A.; Roghanian, R.; Afshar, E.G.; Pardakhty, A.; Mohammadinejad, R.; Kumar, A.; Thakur, V.K. Multifunctional Polymeric Nanoplatforms for Brain Diseases Diagnosis, Therapy and Theranostics. Biomedicines 2020, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Mohammadinejad, R.; Maleki, H.; Larrañeta, E.; Fajardo, A.R.; Nik, A.B.; Shavandi, A.; Sheikhi, A.; Ghorbanpour, M.; Farokhi, M.; Govindh, P. Status and future scope of plant-based green hydrogels in biomedical engineering. Appl. Mater. Today 2019, 16, 213–246. [Google Scholar] [CrossRef] [Green Version]
- Thakur, V.K.; Voicu, S.I. Recent advances in cellulose and chitosan based membranes for water purification: A concise review. Carbohyd. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Alsarra, I.A.; Neau, S.H. An in vitro evaluation of a chitosan-containing multiparticulate system for macromolecule delivery to the colon. Int. J. Pharm 2002, 239, 197–205. [Google Scholar] [CrossRef]
- Saikia, C.; Gogoi, P. Chitosan: A Promising Biopolymer in Drug Delivery Applications. J. Mol. Genet. Med. 2015, s4. [Google Scholar] [CrossRef]
- Jain, D.; Bar-Shalom, D. Alginate drug delivery systems: Application in context of pharmaceutical and biomedical research. Drug Dev. Ind. Pharm. 2014, 40, 1576–1584. [Google Scholar] [CrossRef]
- Yu, C.Y.; Yin, B.C.; Zhang, W.; Cheng, S.X.; Zhang, X.Z.; Zhuo, R.X. Composite microparticle drug delivery systems based on chitosan, alginate and pectin with improved pH-sensitive drug release property. Colloids Surf. B Biointerfaces 2009, 68, 245–249. [Google Scholar] [CrossRef]
- Mulia, K.; Halimah, N.; Krisanti, E. Effect of alginate composition on profile release and characteristics of chitosan-alginate microparticles loaded with mangosteen extract. Aip Conf. Proc. 2017, 1823. [Google Scholar] [CrossRef] [Green Version]
- Soliman, E.A.; El-Moghazy, A.Y.; El-Din, M.S.M.; Massoud, M.A. Microencapsulation of Essential Oils within Alginate: Formulation and in Vitro Evaluation of Antifungal Activity. J. Encapsulation Adsorpt. Sci. 2013, 3, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Jeganathan, P.M.; Venkatachalam, S.; Karichappan, T.; Ramasamy, S. Model development and process optimization for solvent extraction of polyphenols from red grapes using box-behnken design. Prep. Biochem. Biotechnol. 2014, 44, 56–67. [Google Scholar] [CrossRef]
- Bi, W.; Tian, M.; Row, K.H. Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J. Chromatogr. A 2013, 1285, 22–30. [Google Scholar] [CrossRef]
- Yuan, X.; Zeng, Y.; Nie, K.; Wang, Z. Extraction Optimization, Characterizatuib and Bioactives of a Major Polysaccharide from Sargassum thunbergii. PLoS ONE 2015, 0, e0144773. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Park, W.H.; Ha, W.S. Polyelectrolyte complexes of sodium alginate with chitosan or its derivatives for microcapsules. J. Appl. Polym. Sci. 1997, 63, 425–432. [Google Scholar] [CrossRef]
- Yao, K.D.; Peng, T.; Feng, H.B.; He, Y.Y. Swelling kinetics and release characteristic of crosslinked chitosan: Polyether polymer network (semi-IPN) hydrogels. J. Polym. Sci. A Polym. Chem. 1994, 32, 1213–1223. [Google Scholar] [CrossRef]
- Mulia, K.; Andrie; Krisanti, E.A. Effect of sodium tripolyphosphate concentration and simulated gastrointestinal fluids on release profile of paracetamol from chitosan microsphere. Iop Conf. Ser. 2018, 316, 012028. [Google Scholar] [CrossRef] [Green Version]
- Montgomery, D.C. Response Surface Methods and Designs. In Design and Analysis of Experiments, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]




| Level | Microparticle Size (µm) (A) | Alginate: Chitosan Mass Ratio (B) | CaCl2 Concentration (% w/v) (C) |
|---|---|---|---|
| Low (−1) | <100 | 0.10 | 4 |
| Medium (0) | 100–199 | 0.25 | 6 |
| High (1) | 200–299 | 0.50 | 8 |
| Formula | Microparticle Size in µm (A) | Alginate: Chitosan Mass Ratio (B) | [CaCl2] in %-w/v (C) | Cumulative Release (%) | |
|---|---|---|---|---|---|
| expt. | calc. | ||||
| F10 | 0 | 1 | −1 | 97.5 | 97.2 |
| F3 | −1 | 1 | 0 | 96.9 | 96.7 |
| F4 | 1 | 1 | 0 | 83.9 | 83.0 |
| F12 | 0 | 1 | 1 | 83.0 | 84.5 |
| F7 | −1 | 0 | 1 | 71.3 | 70.0 |
| F5 | −1 | 0 | −1 | 61.1 | 61.7 |
| F6 | 1 | 0 | −1 | 60.9 | 62.2 |
| F15 | 0 | 0 | 0 | 59.0 | 59.5 |
| F13 | 0 | 0 | 0 | 60.6 | 59.5 |
| F14 | 0 | 0 | 0 | 58.9 | 59.5 |
| F11 | 0 | −1 | 1 | 54.3 | 54.6 |
| F8 | 1 | 0 | 1 | 49.4 | 48.8 |
| F1 | −1 | −1 | 0 | 52.3 | 53.3 |
| F9 | 0 | −1 | −1 | 48.6 | 47.1 |
| F2 | 1 | −1 | 0 | 46.1 | 46.4 |
| Source | p-Value |
|---|---|
| Model | <0.0001 |
| Microparticle size | 0.0002 |
| Alginate:chitosan mass ratio | <0.0001 |
| CaCl2 concentration | 0.0717 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulia, K.; Singarimbun, A.C.; Krisanti, E.A. Optimization of Chitosan–Alginate Microparticles for Delivery of Mangostins to the Colon Area Using Box–Behnken Experimental Design. Int. J. Mol. Sci. 2020, 21, 873. https://doi.org/10.3390/ijms21030873
Mulia K, Singarimbun AC, Krisanti EA. Optimization of Chitosan–Alginate Microparticles for Delivery of Mangostins to the Colon Area Using Box–Behnken Experimental Design. International Journal of Molecular Sciences. 2020; 21(3):873. https://doi.org/10.3390/ijms21030873
Chicago/Turabian StyleMulia, Kamarza, Ameninta Cesanina Singarimbun, and Elsa Anisa Krisanti. 2020. "Optimization of Chitosan–Alginate Microparticles for Delivery of Mangostins to the Colon Area Using Box–Behnken Experimental Design" International Journal of Molecular Sciences 21, no. 3: 873. https://doi.org/10.3390/ijms21030873