Polydeoxyribonucleotide Exerts Protective Effect Against CCl4-Induced Acute Liver Injury Through Inactivation of NF-κB/MAPK Signaling Pathway in Mice
Abstract
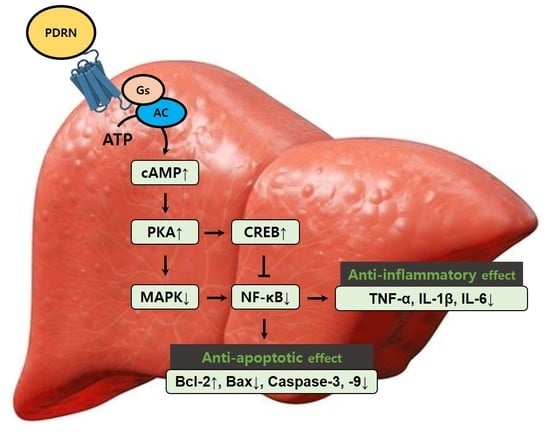
:1. Introduction
2. Results
2.1. Level of AST and ALT, Expression of CYP2E1 and UPC2
2.2. Liver Morphology and Histopathological Score
2.3. Level of Pro-Inflammatory Cytokines
2.4. NF-κB Activation and MAPK Signaling Pathway
2.5. cAMP Concentration, P-CREB vs. CREB Ratio, and P-PKA vs. PKA Ratio
2.6. Cleaved Caspase-3 and Cleaved Caspase-9 Expression, and Bax vs. Bcl-2 Ratio
3. Discussion
4. Methods
4.1. Animals and Classification
4.2. Inducing Acute Liver Injury using CCl4 and Treatment
4.3. Blood Sampling, Liver Index, and Tissue Preparation
4.4. Hematoxylin and Eosin (H&E) Staining and Liver Injury Score Analysis
4.5. Concentration of ALT and AST
4.6. Concentration of Pro-Inflammatory Cytokines and cAMP
4.7. Western Blot Analysis
4.8. Data Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALI | Acute liver injury |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B-cell lymphoma-2 |
| cAMP | cyclic adenosine-3′,5′-monophosphate |
| CCl4 | Carbon tetrachloride |
| CREB | cAMP response element-binding protein |
| CYP2E1 | cytochrome P450 2E1 |
| DMPX | 7-dimethyl-1-propargylxanthine |
| ELISA | Enzyme-linked immunoassay |
| ERK | Extracellular signal-regulated kinase |
| H&E | Hematoxylin and eosin |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| IκB-α | Nuclear factor-κB inhibitor-α |
| JNK | c-Jun N-terminal kinase |
| MAPK | Mitogen-activated protein kinases |
| NF-κB | Nuclear factor-κB |
| PDRN | Polydeoxyribonucleotide |
| PKA | Protein kinases A |
| TNF-α | Tumor necrosis factor-α |
| UCP2 | uncoupling protein2 |
References
- Tsai, J.C.; Chiu, C.S.; Chen, Y.C.; Lee, M.S.; Hao, X.Y.; Hsieh, M.T.; Kao, C.P.; Peng, W.H. Hepatoprotective effect of Coreopsis tinctoria flowers against carbon tetrachloride-induced liver damage in mice. BMC Complement. Altern. Med. 2017, 17, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.G.; Zhang, C.; Wang, J.X.; Wang, B.W.; Wang, H.; Zhang, Z.H.; Chen, Y.H.; Lu, Y.; Tao, L.; Wang, J.Q.; et al. Obeticholic acid protects against carbon tetrachloride-induced acute liver injury and inflammation. Toxicol. Appl. Pharmacol. 2017, 314, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Bernal, W.; Auzinger, G.; Dhawan, A.; Wendon, J. Acute liver failure. Lancet 2010, 376, 190–201. [Google Scholar] [CrossRef]
- Lee, W.M.; Stravitz, R.T.; Larson, A.M. Introduction to the revised American Association for the Study of Liver Diseases Position Paper on acute liver failure 2011. Hepatology 2012, 55, 965–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, C.; Xiao, X.; Li, D.; Tun, S.; Wang, Y.; Velkov, T.; Tang, S. Chloroquine ameliorates carbon tetrachloride-induced acute liver injury in mice via the concomitant inhibition of inflammation and induction of apoptosis. Cell Death Dis. 2018, 9, 1164. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Luedde, T. Apoptosis and necroptosis in the liver: A matter of life and death. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 738–752. [Google Scholar] [CrossRef]
- Yang, K.; Zou, Z.; Wu, Y.; Hu, G. MiR-195 suppression alleviates apoptosis and oxidative stress in CCl4-induced ALI in mice by targeting Pim-1. Exp. Mol. Pathol. 2020, 115, 104438. [Google Scholar] [CrossRef]
- Huang, Q.; Bai, F.; Nie, J.; Lu, S.; Lu, C.; Zhu, X.; Zhuo, L.; Lin, X. Didymin ameliorates hepatic injury through inhibition of MAPK and NF-κB pathways by up-regulating RKIP expression. Int. Immunopharmacol. 2017, 42, 130–138. [Google Scholar] [CrossRef]
- Malhi, H.; Guicciardi, M.E.; Gores, G.J. Hepatocyte death: A clear and present danger. Physiol. Rev. 2010, 90, 1165–1194. [Google Scholar] [CrossRef] [Green Version]
- Munakarmi, S.; Chand, L.; Shin, H.B.; Jang, K.Y.; Jeong, Y.J. Indole-3-carbinol derivative DIM mitigates carbon tetrachloride-induced acute liver injury in mice by inhibiting inflammatory response, apoptosis and regulating oxidative stress. Int. J. Mol. Sci. 2020, 21, 2048. [Google Scholar] [CrossRef] [Green Version]
- Niu, X.; Liu, F.; Li, W.; Zhi, W.; Yao, Q.; Zhao, J.; Yang, G.; Wang, X.; Qin, L.; He, Z. Hepatoprotective effect of fraxin against carbon tetrachloride-induced hepatotoxicity in vitro and in vivo through regulating hepatic antioxidant, inflammation response and the MAPK-NF-κB signaling pathway. Biomed. Pharmacother. 2017, 95, 1091–1102. [Google Scholar] [CrossRef]
- Ko, I.G.; Hwang, J.J.; Chang, B.S.; Kim, S.H.; Jin, J.J.; Hwang, L.; Kim, C.J.; Choi, C.W. Polydeoxyribonucleotide ameliorates lipopolysaccharide-induced acute lung injury via modulation of the MAPK/NF-κB signaling pathway in rats. Int. Immunopharmacol. 2020, 83, 106444. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, T.; Deng, Y.; Hou, L.; Fan, X.; Lin, L.; Zhao, W.; Jiang, K.; Sun, C. Genipin Ameliorates Carbon Tetrachloride-Induced Liver Injury in Mice via the Concomitant Inhibition of Inflammation and Induction of Autophagy. Oxid. Med. Cell. Longev. 2019, 3729051. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [Green Version]
- Cuschieri, J.; Maier, R.V. Mitogen-activated protein kinase (MAPK). Crit. Care Med. 2005, 33, 417–419. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, D.; Ma, S.; Zhao, X.; Wang, S.; Wei, G.; Wang, X.; Wen, A.; Wang, J. Protective effect of wedelolactone against CCl4-induced acute liver injury in mice. Int. Immunopharmacol. 2016, 34, 44–52. [Google Scholar] [CrossRef]
- Mo, W.; Wang, C.; Li, J.; Chen, K.; Xia, Y.; Li, S.; Xu, L.; Lu, X.; Wang, W.; Guo, C. Fucosterol protects against concanavalin A-induced acute liver injury: Focus on P38 MAPK/NF-κB pathway activity. Gastroenterol. Res. Pract. 2018, 17, 2824139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez, G.; Sitkovsky, M.V. Targeting G protein-coupled A2a adenosine receptors to engineer inflammation in vivo. Int. J. Biochem. Cell Biol. 2003, 35, 410–414. [Google Scholar] [CrossRef]
- Ko, I.G.; Kim, S.E.; Jin, J.J.; Hwang, L.; Ji, E.S.; Kim, C.J.; Han, J.H.; Hong, I.T.; Kwak, M.S.; Yoon, J.Y.; et al. Combination therapy with polydeoxyribonucleotide and proton pump inhibitor enhances therapeutic effectiveness for gastric ulcer in rats. Life Sci. 2018, 203, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Chung, J.Y.; Ko, I.G.; Kim, S.H.; Jin, J.J.; Hwang, L.; Moon, E.J.; Lee, B.J.; Yi, J.W. Effect of polydeoxyribonucleotide on lipopolysaccharide and sevoflurane-induced postoperative cognitive dysfunction in human neuronal SH-SY5Y cells. Int. Neurourol. J. 2019, 23, S93–S101. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Ko, I.G.; Jin, J.J.; Hwang, L.; Kim, C.J.; Kim, S.H.; Han, J.H.; Jeon, J.W. Polydeoxyribonucleotide exerts therapeutic effect by increasing VEGF and inhibiting inflammatory cytokines in ischemic colitis rats. Biomed. Res. Int. 2020, 21, 2169083. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.F.; Salem, A.A.; Eassawy, M.M. Hepatoprotective effect of grape seed oil against carbon tetrachloride induced oxidative stress in liver of γ-irradiated rat. J. Photochem. Photobiol. B 2016, 160, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Demori, I.; Burlando, B.; Gerdoni, E.; Lanni, A.; Fugassa, E.; Voci, A. Uncoupling protein-2 induction in rat hepatocytes after acute carbon tetrachloride liver injury. J. Cell Physiol. 2008, 216, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Wasmuth, H.E.; Tacke, F.; Trautwein, C. Chemokines in liver inflammation and fibrosis. Semin. Liver Dis. 2010, 30, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-κB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [Green Version]
- Kiso, K.; Ueno, S.; Fukuda, M.; Ichi, I.; Kobayashi, K.; Sakai, T.; Fukui, K.; Kojo, S. The role of Kupffer cells in carbon tetrachloride intoxication in mice. Biol. Pharm. Bull. 2012, 35, 980–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Scheidereit, C. IκB kinase complexes: Gateways to NF-κB activation and transcription. Oncogene 2006, 25, 6685–6705. [Google Scholar] [CrossRef] [Green Version]
- Verhelst, K.; Carpentier, I.; Beyaert, R. Regulation of TNF-induced NF-κB activation by different cytoplasmic ubiquitination events. Cytokine Growth Factor Rev. 2011, 22, 277–286. [Google Scholar] [CrossRef]
- Giambelluca, M.S.; Pouliot, M. Early tyrosine phosphorylation events following adenosine A2A receptor in human neutrophils: Identification of regulated pathways. J. Leukoc. Biol. 2017, 102, 829–836. [Google Scholar] [CrossRef] [Green Version]
- Haskó, G.; Linden, J.; Cronstein, B.; Pacher, P. Adenosine receptors: Therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 2008, 7, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Chera, S.; Ghila, L.; Wenger, Y.; Galliot, B. Injury-induced activation of the MAPK/CREB pathway triggers apoptosis-induced compensatory proliferation in hydra head regeneration. Dev. Growth Differ. 2011, 53, 186–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Cederbaum, A.I. Inhibition of autophagy promotes CYP2E1-dependent toxicity in HepG2 cells via elevated oxidative stress, mitochondria dysfunction and activation of p38 and JNK MAPK. Redox Biol. 2013, 5, 552–565. [Google Scholar] [CrossRef] [Green Version]
- Inoue, S.; Browne, G.; Melino, G.; Cohen, G.M. Ordering of caspases in cells undergoing apoptosis by the intrinsic pathway. Cell Death Differ. 2009, 16, 1053–1061. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, M.K.; Mehal, W.Z. Activation of adenosine receptor A2A increases HSC proliferation and inhibits death and senescence by down-regulation of p53 and Rb. Front. Pharmacol. 2014, 5, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, S.P.K.; Khole, S.; Jagadish, N.; Ghosh, D.; Gadgil, V.; Sinkar, V.; Ghaskadbi, S.S. Andrographolide protects liver cells from H2O2 induced cell death by upregulation of Nrf-2/HO-1 mediated via adenosine A2a receptor signaling. Biochim. Biophys. Acta 2016, 1860, 2377–2390. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, N.M.; Wahid, A.; Mohamed, E.M.; Abdel-Aziz, M.A.; Mohafez, O.M.; Bakar, S. New pathways driving the experimental hepatoprotective action of tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl) against acute hepatotoxicity. Biomed. Pharmacother. 2016, 79, 215–221. [Google Scholar] [CrossRef]
- Ding, H.; Fang, M.; Gong, Y.; Li, D.; Zhang, C.; Wen, G.; Wu, C.; Yang, J.; Yang, Y. Smad3 gene C-terminal phosphorylation site mutation aggravates CCl4-induced inflammation in mice. J. Cell. Mol. Med. 2020, 14, 2020. [Google Scholar] [CrossRef]
- Kim, J. Low-intensity tower climbing resistance exercise reduces experimentally induced atopic dermatitis in mice. J. Exerc. Rehabil. 2019, 15, 518–525. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Han, T.; Zhang, W.; Li, W.; Hu, Y.; Lee, S.K. Simulated altitude exercise training damages small intestinal mucosa barrier in the rats. J. Exerc. Rehabil. 2018, 14, 341–348. [Google Scholar] [CrossRef]
- Knodell, R.G.; Ishak, K.G.; Black, W.C.; Chen, T.S.; Craig, R.; Kaplowitz, N.; Kiernan, T.W.; Wollman, J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981, 1, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Timóteo, R.P.; Sales-Campos, H.; Silva, M.V.; da Silva, D.A.A.; Da Silva Catarino, J.; de Sousa, M.A.D.; Júnior, V.R.; Bittencourt, A.C.S.; Carneiro, É.M.; Oliveira, C.J.F. Pemphigus foliaceus patients (Fogo Selvagem) treated with kinesiotherapy presented lower levels of proinflammatory cytokines. J. Exerc. Rehabil. 2019, 15, 460–467. [Google Scholar] [CrossRef] [PubMed]







| Grade | Periportal Bridging Necrosis | Centrilobular Degeneration and Necrosis | Portal Inflammation |
|---|---|---|---|
| 0 | None | None | None portal inflammation |
| 1 | Mild piecemeal necrosis | Mild (ballooning degeneration and/or scattered foci of hepatocellular necrosis in <20% of lobules or nodules) | Mild (sprinkling of inflammatory cells in <20% portal tracts) |
| 2 | Moderate piece necrosis (involves less than 50% of the circumference of most portal tracts) | Moderate (involvement of 20~40% of lobules or nodules) | Moderate (increased inflammatory cells in 20~40% of portal tracts) |
| 3 | Marked piecemeal necrosis (involves more than 50% of the circumference of most portal tracts) | Marked (involvement of 50% of lobules or nodules) | Marked (increased inflammatory cells in 50% of portal tracts) |
| 4 | Marked piecemeal necrosis plus bridging necrosis | Very marked (involvement of >60% of lobules or nodules) | Very marked (dense packing of inflammatory cells in >60% of portal tracts |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, I.-G.; Jin, J.-J.; Hwang, L.; Kim, S.-H.; Kim, C.-J.; Han, J.H.; Lee, S.; Kim, H.I.; Shin, H.P.; Jeon, J.W. Polydeoxyribonucleotide Exerts Protective Effect Against CCl4-Induced Acute Liver Injury Through Inactivation of NF-κB/MAPK Signaling Pathway in Mice. Int. J. Mol. Sci. 2020, 21, 7894. https://doi.org/10.3390/ijms21217894
Ko I-G, Jin J-J, Hwang L, Kim S-H, Kim C-J, Han JH, Lee S, Kim HI, Shin HP, Jeon JW. Polydeoxyribonucleotide Exerts Protective Effect Against CCl4-Induced Acute Liver Injury Through Inactivation of NF-κB/MAPK Signaling Pathway in Mice. International Journal of Molecular Sciences. 2020; 21(21):7894. https://doi.org/10.3390/ijms21217894
Chicago/Turabian StyleKo, Il-Gyu, Jun-Jang Jin, Lakkyong Hwang, Sang-Hoon Kim, Chang-Ju Kim, Jin Hee Han, Seunghwan Lee, Ha Il Kim, Hyun Phil Shin, and Jung Won Jeon. 2020. "Polydeoxyribonucleotide Exerts Protective Effect Against CCl4-Induced Acute Liver Injury Through Inactivation of NF-κB/MAPK Signaling Pathway in Mice" International Journal of Molecular Sciences 21, no. 21: 7894. https://doi.org/10.3390/ijms21217894