Opportunities for Antibody Discovery Using Human Pluripotent Stem Cells: Conservation of Oncofetal Targets
Abstract
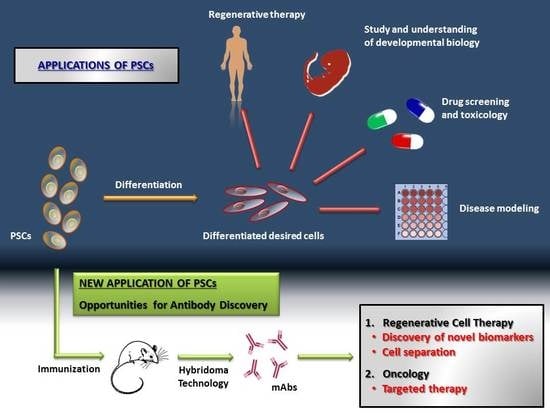
:1. Introduction
2. mAbs for Regenerative Cell Therapy
3. mAbs for Oncology
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Dakhore, S.; Nayer, B.; Hasegawa, K. Human pluripotent stem cell culture: Current status, challenges, and advancement. Stem Cells Int. 2018, 2018, 7396905. [Google Scholar] [CrossRef] [PubMed]
- Seong Gyu, K.; Yang Woo, K.; Tae Wook, L.; Gyu Tae, P.; Jae Ho, K. Recent advances in stem cell therapeutics and tissue engineering strategies. Biomater. Res. 2018, 22, 1–8. [Google Scholar]
- Reubinoff, B.E.; Pera, M.F.; Fong, C.-Y.; Trounson, A.; Bongso, A. Embryonic stem cell lines from human blastocysts: Somatic differentiation in vitro. Nat. Biotechnol. 2000, 18, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S. Induction of pluripotent stem cells from mouse fibroblasts by four transcription factors. Cell Prolif. 2007, 41, 51–56. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Skyler, J.S. Hope vs hype: Where are we in type 1 diabetes? Diabetologia 2018, 61, 509–516. [Google Scholar] [CrossRef]
- Nagoshi, N.; Okano, H. iPSC-derived neural precursor cells: Potential for cell transplantation therapy in spinal cord injury. Cell Mol. Life Sci. 2018, 75, 989–1000. [Google Scholar] [CrossRef]
- Ortiz-Vitali, J.; Darabi, R. iPSCs as a platform for disease modeling, drug screening, and personalized therapy in muscular dystrophies. Cells 2019, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Magli, A.; Perlingeiro, R.R.C. Myogenic progenitor specification from pluripotent stem cells. Semin. Cell Dev. Biol. 2017, 72, 87–98. [Google Scholar] [CrossRef]
- Narazaki, G.; Uosaki, H.; Teranishi, M.; Okita, K.; Kim, B.; Matsuoka, S.; Yamanaka, S.; Yamashita, J.K. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation 2008, 118, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Horisawa, K.; Suzuki, A. Cell-Based regenerative therapy for liver disease. In Innovative Medicine; Springer: Tokyo, Japan, 2015; pp. 327–339. ISBN 9784431556503. [Google Scholar]
- Bernarreggi, D.; Pouyanfard, S.; Kaufman, D.S. Development of innate immune cells from human pluripotent stem cells. Exp. Hematol. 2019, 71, 13–23. [Google Scholar] [CrossRef]
- Montel-Hagen, A.; Crooks, G.M. From pluripotent stem cells to T cells. Exp. Hematol. 2019, 71, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Guhr, A.; Kobold, S.; Seltmann, S.; Seiler Wulczyn, A.E.M.; Kurtz, A.; Löser, P. Recent trends in research with human pluripotent stem cells: Impact of research and use of cell lines in experimental research and clinical trials. Stem Cell Rep. 2018, 11, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Angelos, M.G.; Kaufman, D.S. Pluripotent stem cell applications for regenerative medicine. Curr. Opin. Organ Transpl. 2015, 25, 1. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, E.; Thomas, R.J.; Williams, D.J. Current understanding and challenges in bioprocessing of stem cell-based therapies for regenerative medicine. Br. Med. Bull. 2011, 100, 137–155. [Google Scholar] [CrossRef]
- Abbasalizadeh, S.; Baharvand, H. Technological progress and challenges towards cGMP manufacturing of human pluripotent stem cells based therapeutic products for allogeneic and autologous cell therapies. Biotechnol. Adv. 2013, 31, 1600–1623. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, M.K.; Frey-Vasconcells, J.; Rao, M.S. Developing safe therapies from human pluripotent stem cells. Nat. Biotechnol. 2009, 27, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Kim, W.T.; Ryu, C.J. Antibody approaches to prepare clinically transplantable cells from human embryonic stem cells: Identification of human embryonic stem cell surface markers by monoclonal antibodies. Biotechnol. J. 2014, 9, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Fox, N.W.; Damjanov, I.; Knowles, B.B.; Solter, D. Stage-specific embryonic antigen 3 as a marker of visceral extraembryonic endoderm. Dev. Biol. 1984, 103, 263–266. [Google Scholar] [CrossRef]
- Kannagi, R.; Cochran, N.A.; Ishigami, F.; Hakomori, S.; Andrews, P.W.; Knowles, B.B.; Solter, D. Stage-Specific embryonic antigens (SSEA-3 and -4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 1983, 2, 2355–2361. [Google Scholar] [CrossRef] [PubMed]
- Kannagi, R.; Levery, S.B.; Ishigami, F.; Hakomori, S.; Shevinsky, L.H.; Knowles, B.B.; Solter, D. New globoseries glycosphingolipids in human teratocarcinoma reactive with the monoclonal antibody directed to a developmentally regulated antigen, stage-specific embryonic antigen 3. J. Biol. Chem. 1983, 258, 8934–8942. [Google Scholar] [PubMed]
- Andrews, P.W.; Fenderson, B.; Hakomori, S. Human embryonal carcinoma cells and their differentiation in culture. Int. J. Androl. 1987, 10, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Badcock, G.; Pigott, C.; Goepel, J.; Andrews, P.W. The human embryonal carcinoma marker antigen TRA-1-60 is a sialylated keratan sulfate proteoglycan. Cancer Res. 1999, 59, 4715–4719. [Google Scholar] [PubMed]
- Choi, H.S.; Kim, H.; Won, A.; Kim, J.J.; Son, C.Y.; Kim, K.S.; Ko, J.H.; Lee, M.Y.; Kim, C.H.; Ryu, C.J. Development of a decoy immunization strategy to identify cell-surface molecules expressed on undifferentiated human embryonic stem cells. Cell Tissue Res. 2008, 333, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-J.; Choi, H.S.; Lee, M.-Y.; Ryu, C.J. Characterization of Monoclonal Antibodies Recognizing 130 kDa Surface Proteins on Human Embryonic Stem Cells and Cancer Cell Lines. Monoclon. Antib. Immunodiagn. Immunother. 2013, 32, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.S.; Seong, R.H.; Ryu, C.J.; Cho, Y.S.; Bae, K.-H.; Chung, S.J.; Lee, B.; Min, J.-K.; Hong, H.J. Brief report: L1 cell adhesion molecule, a novel surface molecule of human embryonic stem cells, is essential for self-renewal and pluripotency. Stem Cells 2011, 29, 2094–2099. [Google Scholar] [CrossRef]
- Park, J.; Son, Y.; Lee, N.G.; Lee, K.; Lee, D.G.; Song, J.; Lee, J.; Kim, S.; Cho, M.J.; Jang, J.-H.; et al. DSG2 is a functional cell surface marker for identification and isolation of human pluripotent stem cells. Stem Cell Rep. 2018, 11, 115–127. [Google Scholar] [CrossRef]
- Son, Y.S.; Park, J.H.; Kang, Y.K.; Park, J.-S.; Choi, H.S.; Lim, J.Y.; Lee, J.E.; Lee, J.B.; Ko, M.S.; Kim, Y.; et al. Heat shock 70-kDa protein 8 isoform 1 is expressed on the surface of human embryonic stem cells and downregulated upon differentiation. Stem Cells 2005, 23, 1502–1513. [Google Scholar] [CrossRef] [PubMed]
- Choo, A.B.; Tan, H.L.; Ang, S.N.; Fong, W.J.; Chin, A.; Lo, J.; Zheng, L.; Hentze, H.; Philp, R.J.; Oh, S.K.W.; et al. Selection against undifferentiated human embryonic stem cells by a cytotoxic antibody recognizing podocalyxin-like protein-1. Stem Cells 2008, 26, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Fong, W.J.; Lee, E.H.; Yap, M.; Choo, A. mAb 84, a cytotoxic antibody that kills undifferentiated human embryonic stem cells via oncosis. Stem Cells 2009, 27, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.Y.; Ang, S.N.; Chan, J.X.; Choo, A.B.H. Characterization of epithelial cell adhesion molecule as a surface marker on undifferentiated human embryonic stem cells. Stem Cells 2010, 28, 29–35. [Google Scholar] [CrossRef]
- Cua, S.; Tan, H.L.; Fong, W.J.; Chin, A.; Lau, A.; Ding, V.; Song, Z.; Yang, Y.; Choo, A. Targeting of embryonic annexin A2 expressed on ovarian and breast cancer by the novel monoclonal antibody 2448. Oncotarget 2018, 9, 13206–13221. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Yong, C.; Tan, B.Z.; Fong, W.J.; Padmanabhan, J.; Chin, A.; Ding, V.; Lau, A.; Zheng, L.; Bi, X.; et al. Conservation of oncofetal antigens on human embryonic stem cells enables discovery of monoclonal antibodies against cancer. Sci. Rep. 2018, 8, 11608. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.Y.; Tan, H.L.; Matsudaira, P.T.; Choo, A. Excess reactive oxygen species production mediates monoclonal antibody-induced human embryonic stem cell death via oncosis. Cell Death Differ 2017, 24, 546–558. [Google Scholar] [CrossRef] [Green Version]
- Choo, M.; Tan, H.L.; Ding, V.; Castangia, R.; Belgacem, O.; Liau, B.; Hartley-Tassell, L.; Haslam, S.M.; Dell, A.; Choo, A. Characterization of H type 1 and type 1 N-acetyllactosamine glycan epitopes on ovarian cancer specifically recognized by the anti-glycan monoclonal antibody mAb-A4. J. Biol. Chem. 2017, 292, 6163–6176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schriebl, K.; Satianegara, G.; Hwang, A.; Tan, H.L.; Fong, W.J.; Yang, H.H.; Jungbauer, A.; Choo, A. Selective Removal of Undifferentiated Human Embryonic Stem Cells Using Magnetic Activated Cell Sorting Followed by a Cytotoxic Antibody. Tissue Eng Part A 2012, 18, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, U.; Benvenisty, N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat. Rev Cancer 2011, 11, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, R.; Hamidieh, A.A.; Verdi, J.; Shoae-Hassani, A. Safe Transplantation Of Pluripotent Stem Cell By Preventing Teratoma Formation. J. Stem Cell Res. 2014, 4, 1000212. [Google Scholar] [CrossRef] [Green Version]
- Hentze, H.; Soong, P.L.; Wang, S.T.; Phillips, B.W.; Putti, T.C.; Dunn, N.R. Teratoma formation by human embryonic stem cells: Evaluation of essential parameters for future safety studies. Stem Cell Res. 2009, 2, 198–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujikawa, T.; Oh, S.-H.; Pi, L.; Hatch, H.M.; Shupe, T.; Petersen, B.E. Teratoma formation leads to failure of treatment for type I diabetes using embryonic stem cell-derived insulin-producing cells. Am J. Pathol. 2005, 166, 1781–1791. [Google Scholar] [CrossRef] [Green Version]
- Alper, J. Geron gets green light for human trial of ES cell–derived product. Nat. Biotechnol. 2009, 27, 213–214. [Google Scholar] [CrossRef] [PubMed]
- Amariglio, N.; Hirshberg, A.; Scheithauer, B.W.; Cohen, Y.; Loewenthal, R.; Trakhtenbrot, L.; Paz, N.; Koren-Michowitz, M.; Waldman, D.; Leider-Trejo, L.; et al. Donor-Derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009, 6, e1000029. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, D.B.; Beck, S.G.; Wharram, B.L.; Wiggins, J.E.; Goyal, M.; Thomas, P.E.; Wiggins, R.C. Molecular cloning and characterization of human podocalyxin-like protein. Orthologous relationship to rabbit PCLP1 and rat podocalyxin. J. Biol. Chem. 1997, 272, 15708–15714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandenberger, R.; Wei, H.; Zhang, S.; Lei, S.; Murage, J.; Fisk, G.J.; Li, Y.; Xu, C.; Fang, R.; Guegler, K.; et al. Transcriptome characterization elucidates signaling networks that control human ES cell growth and differentiation. Nat. Biotechnol. 2004, 22, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Schopperle, W.M.; DeWolf, W.C. The TRA-1-60 and TRA-1-81 human pluripotent stem cell markers are expressed on podocalyxin in embryonal carcinoma. Stem Cells 2006, 25, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Nakao, H.; Kawabe, K.; Nonaka, M.; Toyoda, H.; Takishima, Y.; Kawabata, K.; Yamaguchi, T.; Furue, M.K.; Taki, T.; et al. A cytotoxic antibody recognizing lacto- N -fucopentaose I ( LNFP I ) on human induced pluripotent stem ( hiPS ) Cells *. J. Biol. Chem. 2015, 290, 20071–20085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, C.; Lee, A.S.; Volkmer, J.-P.; Sahoo, D.; Nag, D.; Mosley, A.R.; Inlay, M.A.; Ardehali, R.; Chavez, S.L.; Pera, R.R.; et al. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat. Biotechnol. 2011, 29, 829–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, H.L.; Tan, B.Z.; Goh, W.X.T.; Cua, S.; Choo, A. In vivo surveillance and elimination of teratoma-forming human embryonic stem cells with monoclonal antibody 2448 targeting annexin A2. Biotechnol. Bioeng. 2019, 116, 2996–3005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murayama, Y.; Oritani, K.; Tsutsui, S. Novel CD9-targeted therapies in gastric cancer. World J. Gastroenterol. 2015, 21, 3206–3213. [Google Scholar] [CrossRef]
- Liang, P.; Miao, M.; Liu, Z.; Wang, H.; Jiang, W.; Ma, S.; Li, C.; Hu, R. CD9 expression indicates a poor outcome in acute lymphoblastic leukemia. Cancer Biomark. 2018, 21, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Wachowiak, R.; Krause, M.; Mayer, S.; Peukert, N.; Suttkus, A.; Müller, W.C.; Lacher, M.; Meixensberger, J.; Nestler, U. Increased L1CAM (CD171) levels are associated with glioblastoma and metastatic brain tumors. Medicine 2018, 97, e12396. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-C.; Xie, Y.-M.; Ran, L.-Q.; Cao, H.-H.; Sun, C.; Wu, J.-Y.; Wu, Z.-Y.; Liao, L.-D.; Zhao, W.-J.; Fang, W.-K.; et al. L1CAM drives oncogenicity in esophageal squamous cell carcinoma by stimulation of ezrin transcription. J. Mol. Med. 2017, 95, 1355–1368. [Google Scholar] [CrossRef] [PubMed]
- Han, C.-P.; Yu, Y.-H.; Wang, A.-G.; Tian, Y.; Zhang, H.-T.; Zheng, Z.-M.; Liu, Y.-S. Desmoglein-2 overexpression predicts poor prognosis in hepatocellular carcinoma patients. Eur. Rev. Med. Pharm. Sci. 2018, 22, 5481–5489. [Google Scholar]
- Brennan, D.; Mahoney, M.G. Increased expression of Dsg2 in malignant skin carcinomas: A tissue-microarray based study. Cell Adh. Migr. 2009, 3, 148–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, Z.; Shervington, A.; Munje, C.; Shervington, L. The complexity of identifying cancer stem cell biomarkers. Cancer Invest. 2013, 31, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Brewer, B.G.; Mitchell, R.A.; Harandi, A.; Eaton, J.W. Embryonic vaccines against cancer: An early history. Exp. Mol. Pathol. 2009, 86, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zeng, H.; Xu, R.H.; Liu, B.; Li, Z. Vaccination with human pluripotent stem cells generates a broad spectrum of immunological and clinical responses against colon cancer. Stem Cells 2009, 27, 3103–3111. [Google Scholar] [CrossRef]
- Dong, W.; Qiu, C.; Shen, H.; Liu, Q.; Du, J. Antitumor effect of embryonic stem cells in a non-small cell lung cancer model: Antitumor factors and immune responses. Int. J. Med. Sci. 2013, 10, 1314–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, A.J.; Andrews, P.W. Surface marker antigens in the characterization of human embryonic stem cells. Stem Cell Res. 2009, 3, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Magdelénat, H. Tumour markers in oncology: Past, present and future. J. Immunol. Methods 1992, 150, 133–143. [Google Scholar] [CrossRef]
- Carter, P.; Smith, L.; Ryan, M. Identification and validation of cell surface antigens for antibody targeting in oncology. Endocr. Relat. Cancer 2004, 11, 659–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamandis, E.P.; Bast, R.C.; Gold, P.; Chu, T.M.; Magnani, J.L. Reflection on the discovery of carcinoembryonic antigen, prostate-specific antigen, and cancer antigens CA125 and CA19-9. Clin. Chem. 2013, 59, 22–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graves, M.L.; Cipollone, J.A.; Austin, P.; Bell, E.M.; Nielsen, J.S.; Gilks, C.B.; McNagny, K.M.; Roskelley, C.D. The cell surface mucin podocalyxin regulates collective breast tumor budding. Breast Cancer Res. 2016, 18, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amo, L.; Tamayo-Orbegozo, E.; Maruri, N.; Buqué, A.; Solaun, M.; Riñón, M.; Arrieta, A.; Larrucea, S. Podocalyxin-like protein 1 functions as an immunomodulatory molecule in breast cancer cells. Cancer Lett. 2015, 368, 26–35. [Google Scholar] [CrossRef]
- Snyder, K.A.; Hughes, M.R.; Hedberg, B.; Brandon, J.; Hernaez, D.C.; Bergqvist, P.; Cruz, F.; Po, K.; Graves, M.L.; Turvey, M.E.; et al. Podocalyxin enhances breast tumor growth and metastasis and is a target for monoclonal antibody therapy. Breast Cancer Res. 2015, 17, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Borg, D.; Hedner, C.; Nodin, B.; Larsson, A.; Johnsson, A.; Eberhard, J.; Jirström, K. Expression of podocalyxin-like protein is an independent prognostic biomarker in resected esophageal and gastric adenocarcinoma. BMC Clin. Pathol. 2016, 16, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laitinen, A.; Bockelman, C.; Hagstrom, J.; Kokkola, A.; Fermér, C.; Nilsson, O.; Haglund, C. Podocalyxin as a prognostic marker in gastric cancer. PLoS ONE 2015, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kaprio, T.; Fermér, C.; Hagström, J.; Mustonen, H.; Böckelman, C.; Nilsson, O.; Haglund, C. Podocalyxin is a marker of poor prognosis in colorectal cancer. BMC Cancer 2014, 14, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kusumoto, H.; Shintani, Y.; Kanzaki, R.; Kawamura, T.; Funaki, S.; Minami, M.; Nagatomo, I.; Morii, E.; Okumura, M. Podocalyxin influences malignant potential by controlling epithelial–mesenchymal transition in lung adenocarcinoma. Cancer Sci. 2017, 108, 528–535. [Google Scholar] [CrossRef] [Green Version]
- Jirström, K.; Ahlgren, G.; Andersson, G.; Nodin, B.; Wennersten, C.; Boman, K. Podocalyxin-like and RNA-binding motif protein 3 are prognostic biomarkers in urothelial bladder cancer: A validatory study. Biomark Res. 2017, 5, 1–10. [Google Scholar]
- Taniuchi, K.; Furihata, M.; Naganuma, S.; Dabanaka, K.; Hanazaki, K.; Saibara, T. Podocalyxin-Like protein, linked to poor prognosis of pancreatic cancers, promotes cell invasion by binding to gelsolin. Cancer Sci. 2016, 107, 1430–1442. [Google Scholar] [CrossRef] [PubMed]
- Modjtahedi, H.; Ali, S.; Essapen, S. Therapeutic application of monoclonal antibodies in cancer: Advances and challenges. Br. Med. Bull. 2012, 104, 41–59. [Google Scholar] [CrossRef] [Green Version]
- Scott, A.M.; Allison, J.P.; Wolchok, J.D. Monoclonal antibodies in cancer therapy. Cancer Immun. 2012, 12, 14. [Google Scholar] [PubMed]
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody therapy of cancer. Nat. Rev Cancer 2012, 12, 278–287. [Google Scholar] [CrossRef]
- Pillay, V.; Gan, H.K.; Scott, A.M. Antibodies in oncology. N. Biotechnol. 2011, 28, 518–529. [Google Scholar] [CrossRef]
- Weiner, G.J. Building better monoclonal antibody-based therapeutics. Nat. Rev. Cancer 2015, 15, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Wold, E.D.; Smider, V.V.; Felding, B.H. Antibody therapeutics in oncology. Immunother. Open Access 2016, 2, 1–18. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Y.; Gu, J.; Schlossman, S.F. A cell surface receptor defined by a mAb mediates a unique type of cell death similar to oncosis. Proc. Natl. Acad. Sci. USA 2002, 95, 6290–6295. [Google Scholar] [CrossRef] [Green Version]
- Loo, D.; Pryer, N.; Young, P.; Liang, T.; Coberly, S.; King, K.L.; Kang, K.; Roberts, P.; Tsao, M.; Xu, X.; et al. The glycotope-specific RAV12 monoclonal antibody induces oncosis in vitro and has antitumor activity against gastrointestinal adenocarcinoma tumor xenografts in vivo. Mol. Cancer 2007, 6, 856–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohashi, R.; Kawahara, K.; Fujii, T.; Takei, H.; Naito, Z. Higher expression of EpCAM is associated with poor clinical and pathological responses in breast cancer patients undergoing neoadjuvant chemotherapy. Pathol. Int. 2016, 66, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, S.; Hojati, Z.; Tabatabaeian, H. Cooverexpression of EpCAM and c-myc genes in malignant breast tumours. J. Genet. 2017, 96, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, X.; Gao, J.; Sun, Y.; Liu, T.; Yan, Q.; Yang, X. The role of epithelial cell adhesion molecule N-glycosylation on apoptosis in breast cancer cells. Tumor Biol. 2017, 39, 101042831769597. [Google Scholar] [CrossRef] [Green Version]
- Battista, M.J.; Cotarelo, C.; Jakobi, S.; Steetskamp, J.; Makris, G.; Sicking, I.; Weyer, V.; Schmidt, M. Overexpression of epithelial cell adhesion molecule protein is associated with favorable prognosis in an unselected cohort of ovarian cancer patients. J. Cancer Res. Clin. Oncol. 2014, 140, 1097–1102. [Google Scholar] [CrossRef]
- Woopen, H.; Pietzner, K.; Richter, R.; Fotopoulou, C.; Joens, T.; Braicu, E.I.; Mellstedt, H.; Mahner, S.; Lindhofer, H.; Darb-Esfahani, S.; et al. Overexpression of the epithelial cell adhesion molecule is associated with a more favorable prognosis and response to platinum-based chemotherapy in ovarian cancer. J. Gynecol. Oncol. 2014, 25, 221–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.; Ramjeesingh, R.; Chen, C.H.; Hurlbut, D.; Hammad, N.; Mulligan, L.M.; Nicol, C.; Feilotter, H.E.; Davey, S. Reduction in membranous immunohistochemical staining for the intracellular domain of epithelial cell adhesion molecule correlates with poor patient outcome in primary colorectal adenocarcinoma. Curr. Oncol. 2016, 23, e171–e178. [Google Scholar] [CrossRef] [Green Version]
- Dai, M.; Yuan, F.; Fu, C.; Shen, G.; Hu, S.; Shen, G. Relationship between epithelial cell adhesion molecule (EpCAM) overexpression and gastric cancer patients: A systematic review and meta-analysis. PLoS ONE 2017, 12, 1–15. [Google Scholar] [CrossRef]
- Wang, L.; Schulz, T.C.; Sherrer, E.S.; Dauphin, D.S.; Shin, S.; Nelson, A.M.; Ware, C.B.; Zhan, M.; Song, C.-Z.; Chen, X.; et al. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood 2007, 110, 4111–4119. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, N.; Iqbal, N. Human epidermal growth factor receptor 2 (HER2) in cancers: Overexpression and therapeutic implications. Mol. Biol. Int. 2014, 2014, 852748. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, K.; Yuraszeck, T.; Li, C.C.; Zhang, Y.; Kasichayanula, S. Immunotherapy and Novel Combinations in Oncology: Current Landscape, Challenges, and Opportunities. Clin. Transl Sci. 2016, 9, 89–104. [Google Scholar] [CrossRef]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.J.; Lutz, R.J.; et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, T.; Rowden, G.; Sullivan, A.K.; Pitzele, R. Fetal antigens in nonneoplastic conditions. Cancer Res. 1976, 36, 3446–3452. [Google Scholar]
- Tsuchiya, N. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 10573. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, A.; Utratna, M.; O’Dwyer, M.E.; Joshi, L.; Kilcoyne, M. Glycosylation-Based serum biomarkers for cancer diagnostics and prognostics. BioMed. Res. Int. 2015, 2015, 490531. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-T.; Huang, M.-Y.; Yeh, Y.-S.; Huang, C.-W.; Tsai, H.-L.; Cheng, T.-L.; Wang, J.-Y. A prospective study of comparing multi-gene biomarker chip and serum carcinoembryonic antigen in the postoperative surveillance for patients with stage I–III colorectal cancer. PLoS ONE 2016, 11, e0163264. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Zi, H.; Li, Y.; Gao, Y.; Ge, C.; Sun, Z.; Zhang, Y. Combined use of salivary biomarkers and carcinoembryonic antigen for lung cancer detection in a Chinese population. Medicine 2019, 98, e16511. [Google Scholar] [CrossRef]
- Prager, G.W.; Braemswig, K.H.; Martel, A.; Unseld, M.; Heinze, G.; Brodowicz, T.; Scheithauer, W.; Kornek, G.; Zielinski, C.C. Baseline carcinoembryonic antigen (CEA) serum levels predict bevacizumab-based treatment response in metastatic colorectal cancer. Cancer Sci. 2014, 105, 996–1001. [Google Scholar] [CrossRef]
- Raju, R.; Chau, D.; Cho, D.S.; Park, Y.; Verfaillie, C.M.; Hu, W.-S. Cell expansion during directed differentiation of stem cells toward the hepatic lineage. Stem Cells Dev. 2017, 26, 274–284. [Google Scholar] [CrossRef]
- Ang, L.T.; Tan, A.K.Y.; Autio, M.I.; Goh, S.H.; Choo, S.H.; Lee, K.L.; Tan, J.; Pan, B.; Lee, J.J.H.; Lum, J.J.; et al. A roadmap for human liver differentiation from pluripotent stem cells. Cell Rep. 2018, 22, 2190–2205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitadani, J.; Ojima, T.; Iwamoto, H.; Tabata, H.; Nakamori, M.; Nakamura, M.; Hayata, K.; Katsuda, M.; Miyajima, M.; Yamaue, H. Cancer vaccine therapy using carcinoembryonic antigen—Expressing dendritic cells generated from induced pluripotent stem cells. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Barkeer, S.; Chugh, S.; Batra, S.K.; Ponnusamy, M.P. Glycosylation of cancer stem cells: Function in stemness, tumorigenesis, and metastasis. Neoplasia 2018, 20, 813–825. [Google Scholar] [CrossRef]
- Kim, Y.J.; Varki, A. Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconj. J. 1997, 14, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Sougawa, N.; Miyagawa, S.; Fukushima, S.; Kawamura, A.; Yokoyama, J.; Ito, E.; Harada, A.; Okimoto, K.; Mochizuki-Oda, N.; Saito, A.; et al. Immunologic targeting of CD30 eliminates tumourigenic human pluripotent stem cells, allowing safer clinical application of hiPSC-based cell therapy. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
| mAbs | Immunogen | Antigen Target | Antigen Type 1 | Cells that mAbs Bind 1 | Authors (References) |
|---|---|---|---|---|---|
| L125-C2 | hESC | CD9 | Protein | PSC | Choi et al. [26] |
| 63-B6 | hESC | ND 2 | Protein | PSC, EC, Cancers | Kim et al. [27] |
| 246-D7 | hESC | ND 2 | Protein | PSC, EC, Cancers | Kim et al. [27] |
| 4-63 | hESC | L1CAM | Protein | PSC | Son et al. [28] |
| K6-1 | hESC | DSG2 | Protein | PSC | Park et al. [29] |
| 20-202S | hESC | HSPA8 | Protein | PSC, Cancers | Son et al. [30] |
| mAb 84 | hESC | PODXL | Glycoprotein | PSC, EC | Choo. et al. Tan et al. [31,32] |
| R-17F | iPSC | Lacto-N-fucopentose I | Glycolipid | PSC | Matsumoto. et al. [48] |
| A1 | hESC | Fucα1-2Galβ1-3GlcNAcβ1-3Galβ1 | Glycan | PSC | Zheng et al. (36) |
| mAb-A4 | hESC | Type 1 LacNAc and H Type 1 | Glycan | PSC, Cancers | Choo. et al. [37] |
| SSEA-5 | hESC | H Type 1 | Glycan | PSC | Tang. et al. [49] |
| 2448 | hESC | Annexin A2 | Glycoprotein | PSC, Cancers | Tan et al. [50] |
| mAbs | Immunogen | Antigen Target | Antigen Type 1 | Cells that mAbs Bind 1 | Mechanism of Action (MOA) | Authors (References) |
|---|---|---|---|---|---|---|
| mAb 8 | hESC | EpCAM | Protein | PSC, Cancers | No MOA | Ng et al. [33] |
| 2448 | hESC | Annexin A2 | Glycoprotein | PSC, Cancers | Internalization (ADC), ADCC | Cua et al. [34] |
| A19 | hESC | Erbb-2 | Glycoprotein | PSC, Cancers | Internalization (ADC) | Tan et al. [35] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, H.L.; Choo, A. Opportunities for Antibody Discovery Using Human Pluripotent Stem Cells: Conservation of Oncofetal Targets. Int. J. Mol. Sci. 2019, 20, 5752. https://doi.org/10.3390/ijms20225752
Tan HL, Choo A. Opportunities for Antibody Discovery Using Human Pluripotent Stem Cells: Conservation of Oncofetal Targets. International Journal of Molecular Sciences. 2019; 20(22):5752. https://doi.org/10.3390/ijms20225752
Chicago/Turabian StyleTan, Heng Liang, and Andre Choo. 2019. "Opportunities for Antibody Discovery Using Human Pluripotent Stem Cells: Conservation of Oncofetal Targets" International Journal of Molecular Sciences 20, no. 22: 5752. https://doi.org/10.3390/ijms20225752