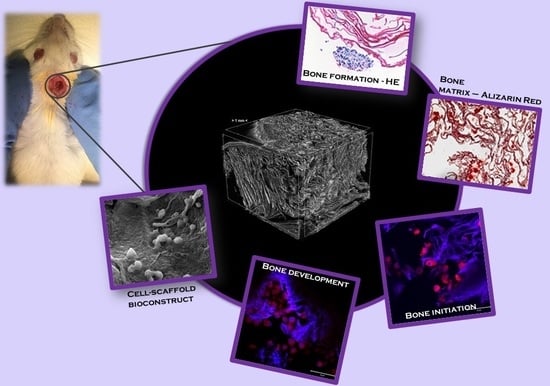
Graphene Oxide Enhances Chitosan-Based 3D Scaffold Properties for Bone Tissue Engineering
Abstract
:1. Introduction
2. Results
2.1. Assessment of CHT/GO Composite Cytocompatibility
2.2. Evaluation of hASC Morphology and Cell Cytoskeleton Organization in BC0.5–BC3
2.3. CHT/GO Materials Characterization by MicroCT
2.4. Cells Distribution and Morphology When Embedded in BC0.5-BC3
2.5. Evolution of the Osteogenic Differentiation Process in BC0.5–BC3
2.5.1. Bone-Like Cell Phenotype and Extracellular Matrix Production in BC0.5–BC3
2.5.2. Evaluation of Osteogenic Differentiation by Histological Staining
2.5.3. Evaluation of Osteogenic Markers Gene Expression
2.5.4. Protein Expression of Osteogenic Markers in BC0.5-3
2.6. In Vivo Evaluation of Bone Regeneration using CHT/GO Materials
3. Discussion
4. Materials and Methods
4.1. Material Preparation
4.2. Material Characterization by MicroCT Analysis
4.3. Cell Culture
4.4. In Vitro Experiments
4.4.1. Biocompatibility Assays
4.4.2. Cytoskeleton Assessment
4.4.3. Cell Morphology Before and During Differentiation by Scanning Electron Microscopy (SEM)
4.4.4. Histological Evaluation of Bone Differentiation
4.4.5. Bone Markers Gene Expression Evaluation by Quantitative PCR (qPCR)
4.4.6. Bone Marker Protein Expression Evaluation by Confocal Microscopy
4.5. In Vivo Experiments
4.5.1. Animals and Experimental Design
4.5.2. Statistical Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.L.; Ahn, J.H.; Hong, B.H.; Pastorin, G.; et al. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef] [PubMed]
- Depan, D.; Misra, R.D. The interplay between nanostructured carbon-grafted chitosan scaffolds and protein adsorption on the cellular response of osteoblasts: Structure function property relationship. Acta Biomater. 2013, 9, 6084–6094. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Liu, Y.; Chen, T.; Du, F.; Zhao, X.; Xiong, C.; Zhou, Y. Is graphene a promising nano-material for promoting surface modification of implants or scaffold materials in bone tissue engineering? Tissue Eng. Part B Rev. 2014, 20, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, Y.; Gao, G.; Chen, J.; Hou, R.; Wang, Q.; Liu, L.; Fu, J. Conductive Graphene Oxide Hydrogels Reduced and Bridged by L-Cysteine to Support Cell Adhesion and Growth. J. Mater. Chem. B 2018, 5, 511–516. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, L.; Liu, M.; Zhang, Z. Biomedical applications of graphene. Theranostics 2012, 2, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Tan, S.; Yu, A.; Zhang, J.; Liu, J.; Mai, W. Sodium 1-naphthalen esulfonate-functionalized reduced graphene oxide stabilizes silver nanoparticles with lower cytotoxicity and long-term antibacterial activity. Chem. Asian J. 2012, 7, 1664–1670. [Google Scholar] [CrossRef]
- Hong, B.J.; Compton, O.C.; An, Z.; Eryazici, I.; Nguyen, S.T. Successful stabilization of graphene oxide in electrolyte solutions: Enhancement of biofunctionalization and cellular uptake. ACS Nano 2012, 6, 63–73. [Google Scholar] [CrossRef]
- Jeong, J.T.; Choi, M.K.; Sim, Y.; Lim, J.T.; Kim, G.S.; Seong, M.J.; Hyung, J.H.; Kim, K.S.; Umar, A.; Lee, S.K. Effect of graphene oxide ratio on the cell adhesion and growth behavior on a graphene oxide-coated silicon substrate. Sci. Rep. 2016, 6, 33835. [Google Scholar] [CrossRef]
- Kenry, L.W.C.; Loh, K.P.; Lim, C.T. When stem cells meet graphene: Opportunities and challenges in regenerative medicine. Biomaterials 2018, 155, 236–250. [Google Scholar] [CrossRef]
- Fu, C.; Bai, H.; Zhu, J.; Niu, Z.; Wang, Y.; Li, J.; Yang, X.; Bai, Y. Enhanced cell proliferation and osteogenetic differentiation in electrospun PLGA/hydroxyapatite nanofibre scaffolds incorporated with graphene oxide. PLoS ONE 2017, 12, e0188352. [Google Scholar] [CrossRef]
- Kang, E.S.; Song, I.; Kim, D.S.; Lee, U.; Kim, J.K.; Son, H.; Min, J.; Kim, T.H. Size-dependent effects of graphene oxide on the osteogenesis of human adipose-derived mesemchymal stem cells. Colloids Surf. B Biointerfaces 2018, 169, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, C.; Yan, J.; Zhang, Q.; Dang, B.; Wang, Z.; Yao, Y.; Lin, K.; Guo, Z.; Bi, L.; et al. Evaluation of the osteogenesis and osteointegration of titanium alloys coated with graphene: An in vivo study. Sci. Rep. 2018, 8, 1843. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y.R.; Kim, Y.; Lim, K.T.; Seonwoo, H.; Park, S. Graphene-incorporated chitosan substrata for adhesion and differentiation of human mesenchymal stem cells. J. Mater. Chem. B 2013, 1, 933–938. [Google Scholar] [CrossRef]
- Lyu, J.Y.; Cao, C.H.; Luo, D.; Fu, Y.X.; He, Y.S.; Zou, D.R. Induction of Osteogenic Differentiation of Human Adipose-Derived Stem Cells by a Novel Self-Supporting Graphene Hydrogel Film and the Possible Underlying Mechanism. ACS Appl. Mater. Interfaces 2015, 7, 20245–20254. [Google Scholar] [CrossRef] [PubMed]
- La, W.G.; Park, S.; Yoon, H.H.; Jeong, G.J.; Lee, T.J.; Bhang, S.H.; Han, J.Y.; Char, K.; Kim, B.S. Delivery of a therapeutic protein for bone regeneration from a substrate coated with graphene oxide. Small 2013, 9, 4051–4060. [Google Scholar] [CrossRef] [PubMed]
- Kalbacova, M.; Broz, A.; Kong, J.; Kalbac, M. Graphene substrates promote adherence of human osteoblasts and mesenchymal stromal cells. Carbon 2010, 48, 4323–4329. [Google Scholar] [CrossRef]
- Some, S.; Ho, S.M.; Dua, P.; Hwang, E.; Shin, Y.H.; Yoo, H.; Kang, J.S.; Lee, D.K.; Lee, H. Dual functions of highly potent graphene derivative-poly-L-lysine composites to inhibit bacteria and support human cells. ACS Nano 2012, 6, 7151–7161. [Google Scholar] [CrossRef]
- Sahu, A.; Choi, W.I.; Tae, G. A stimuli-sensitive injectable graphene oxide composite hydrogel. Chem. Commun. 2012, 48, 5820–5822. [Google Scholar] [CrossRef]
- Ionita, M.; Crica, L.E.; Tiainen, H.; Haugen, H.J.; Vasile, E.; Dinescu, S.; Costache, M.; Iovu, H. Gelatin–poly(vinyl alcohol) porous biocomposites reinforced with graphene oxide as biomaterials. J. Mater. Chem. B 2016, 4, 282–291. [Google Scholar] [CrossRef]
- Pandele, A.M.; Ionita, M.; Crica, L.; Dinescu, S.; Costache, M.; Iovu, H. Synthesis, characterization, and in vitro studies of graphene oxide/chitosan-polyvinyl alcohol films. Carbohydr. Polym. 2014, 102, 813–820. [Google Scholar] [CrossRef]
- Hermenean, A.; Codreanu, A.; Herman, H.; Balta, C.; Rosu, M.; Mihali, C.; Ivan, A.; Dinescu, S.; Ionita, M.; Costache, M. Chitosan-Graphene Oxide 3D scaffolds as Promising Tools for Bone Regeneration in Critical-Size Mouse Calvarial Defects. Sci. Rep. 2017, 7, 16641. [Google Scholar] [CrossRef]
- Lu, J.Y.; He, Y.S.; Cheng, C.; Wang, Y.; Qiu, L.; Li, D.; Zou, D.R. Self-Supporting Graphene Hydrogel Film as an Experimental Platform to Evaluate the Potential of Graphene for Bone Regeneration. Adv. Funct. Mater. 2013, 23, 3494–3502. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, Y.C.; Lee, S.M.; Jin, O.S.; Kang, S.H.; Hong, S.W.; Jeong, C.M.; Huh, J.B.; Han, D.W. Enhanced Osteogenesis by Reduced Graphene Oxide/Hydroxyapatite Nanocomposites. Sci. Rep. 2015, 5, 18833. [Google Scholar] [CrossRef]
- La, W.G.; Jung, M.J.; Yoon, J.K.; Bhang, S.H.; Jang, H.K.; Lee, T.J.; Yoon, H.H.; Shin, J.Y.; Kim, B.S. Bone morphogenetic protein-2 for bone regeneration – Dose reduction through graphene oxide-based delivery. Carbon 2014, 78, 428–438. [Google Scholar] [CrossRef]
- Xie, Y.; Li, H.; Ding, C.; Zheng, X.; Li, K. Effects of graphene plates’ adoption on the microstructure, mechanical properties, and in vivo biocompatibility of calcium silicate coating. Int. J. Nanomed. 2015, 10, 3855–3863. [Google Scholar] [CrossRef]
- Xie, H.; Cao, T.; Gomes, J.V.; Neto, A.H.C.; Rosa, V. Two and three-dimensional graphene substrates to magnify osteogenic differentiation of periodontal ligament stem cells. Carbon 2015, 93, 266–275. [Google Scholar] [CrossRef]
- Ciuffi, S.; Zonefrati, R.; Brandi, M.L. Adipose stem cells for bone tissue repair. Clin. Cases Miner. Bone Metab. 2017, 14, 217–226. [Google Scholar] [CrossRef]
- Cowan, C.M.; Shi, Y.Y.; Aalami, O.O.; Chou, Y.F.; Mari, C.; Thomas, R.; Quarto, N.; Contag, C.H.; Wu, B.; Longaker, M.T. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat. Biotechnol. 2004, 22, 560–567. [Google Scholar] [CrossRef]
- Mesimäki, K.; Lindroos, B.; Törnwall, J.; Mauno, J.; Lindqvist, C.; Kontio, R.; Miettinen, S.; Suuronen, R. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int. J. Oral Maxillofac. Surg. 2009, 38, 201–209. [Google Scholar] [CrossRef]
- Lendeckel, S.; Jödicke, A.; Christophis, P.; Heidinger, K.; Wolff, J.; Fraser, J.K.; Hedrick, M.H.; Berthold, L.; Howaldt, H.P. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: Case report. J. Craniomaxillofac. Surg. 2004, 32, 370–373. [Google Scholar] [CrossRef]
- Dawson, E.; Mapili, G.; Erickson, K.; Taqvi, S.; Roy, K. Biomaterials for stem cell differentiation. Adv. Drug Deliv. Rev. 2008, 60, 215–228. [Google Scholar] [CrossRef]
- Dai, R.; Wang, Z.; Samanipour, R.; Koo, K.I.; Kim, K. Adipose-derived stem cells for tissue engineering and regenerative medicine applications. Stem Cells Int. 2016, 2016, 6737345. [Google Scholar] [CrossRef]
- Burdick, J.A.; Vunjak-Novakovic, G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng. Part A 2009, 15, 205–219. [Google Scholar]
- Hoekstra, A.; Struszczyk, H.; Kivekäs, O. Percutaneous microcrystalline chitosan application for sealing arterial puncture sites. Biomaterials 1998, 19, 1467–1471. [Google Scholar] [CrossRef]
- Gomathysankar, S.; Halim, A.S.; Yaacob, N.S.; Noor, N.M.; Mohamed, M. Compatibility of porous chitosan scaffold with the attachment and proliferation of human adipose-derived stem cells in vitro. J. Stem Cells Regen. Med. 2016, 12, 79–86. [Google Scholar]
- Malafaya, P.B.; Pedro, A.J.; Peterbauer, A.; Gabriel, C.; Redl, H.; Reis, R.L. Chitosan particles agglomerated scaffolds for cartilage and osteochondral tissue engineering approaches with adipose tissue derived stem cells. J. Mater. Sci. Mater. Med. 2005, 16, 1077–1085. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Choi, K.S.; Kim, Y.; Lim, K.T.; Seonwoo, H.; Park, Y.; Kim, D.H.; Choung, P.H.; Cho, C.S.; Kim, S.Y.; et al. Bioactive effects of graphene oxide cell culture substratum on structure and function of human adipose-derived stem cells. J Biomed Mater Res A 2013, 101, 3520–3530. [Google Scholar] [CrossRef]
- Noh, M.; Kim, S.H.; Kim, J.; Lee, J.R.; Jeong, G.J.; Yoon, J.K.; Kang, S.; Bhang, S.H.; Yoon, H.H.; Lee, J.C.; et al. Graphene oxide reinforced hydrogels for osteogenic differentiation of human adipose-derived stem cells. RSC Adv. 2017, 7, 20779–20788. [Google Scholar] [CrossRef] [Green Version]
- Prasadh, S.; Suresh, S.; Wong, R. Osteogenic Potential of Graphene in Bone Tissue Engineering Scaffolds. Materials 2018, 11, 1430. [Google Scholar] [CrossRef]
- Ruan, J.; Wang, X.; Yu, Z.; Wang, Z.; Xie, Q.; Zhang, D.; Huang, Y.; Zhou, H.; Bi, X.; Xiao, C.; et al. Enhanced Physiochemical and Mechanical Performance of Chitosan-Grafted Graphene Oxide for Superior Osteoinductivity. Adv. Funct. Mater. 2016, 26, 1085–1097. [Google Scholar] [CrossRef]
- Ignat, S.R.; Lazăr, A.D.; Şelaru, A.; Samoilă, I.; Vlăsceanu, G.M.; Ioniţă, M.; Radu, E.; Dinescu, S.; Costache, M. Versatile Biomaterial Platform Enriched with Graphene Oxide and Carbon Nanotubes for Multiple Tissue Engineering Applications. Int. J. Mol. Sci. 2019, 20, 3868. [Google Scholar] [CrossRef]
- Dinescu, S.; Ionita, M.; Pandele, A.M.; Galateanu, B.; Iovu, H.; Ardelean, A.; Costache, M.; Hermenean, A. In vitro cytocompatibility evaluation of chitosan/graphene oxide 3D scaffold composites designed for bone tissue engineering. Biomed. Mater. Eng. 2014, 24, 2249–2256. [Google Scholar] [Green Version]
- Ludwig, R. Electron Optics of a Scanning Electron Microscope. In Scanning Electron Microscopy—Physics of Image Formation and Microanalysis; Ludwig, R., Ed.; Springer: Berlin, Germany, 1998; pp. 13–56. [Google Scholar]








| Sample | T.Po (%) | St.Th. (µ) | Sp.S (µ−1) |
|---|---|---|---|
| CH | 74 ± 1.49 | 16.8 ± 2.64 | 0.195 ± 0.03 |
| CH/0.5 wt.% GO | 71.7 ± 1.89 | 20.1 ± 5.54 | 0.169 ± 0.05 |
| CH/3 wt.% GO | 76.4 ± 4.82 | 20.3 ± 4.32 | 0.181 ± 0.08 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinescu, S.; Ionita, M.; Ignat, S.-R.; Costache, M.; Hermenean, A. Graphene Oxide Enhances Chitosan-Based 3D Scaffold Properties for Bone Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 5077. https://doi.org/10.3390/ijms20205077
Dinescu S, Ionita M, Ignat S-R, Costache M, Hermenean A. Graphene Oxide Enhances Chitosan-Based 3D Scaffold Properties for Bone Tissue Engineering. International Journal of Molecular Sciences. 2019; 20(20):5077. https://doi.org/10.3390/ijms20205077
Chicago/Turabian StyleDinescu, Sorina, Mariana Ionita, Simona-Rebeca Ignat, Marieta Costache, and Anca Hermenean. 2019. "Graphene Oxide Enhances Chitosan-Based 3D Scaffold Properties for Bone Tissue Engineering" International Journal of Molecular Sciences 20, no. 20: 5077. https://doi.org/10.3390/ijms20205077