Impact of Serum Source on Human Mesenchymal Stem Cell Osteogenic Differentiation in Culture
Abstract
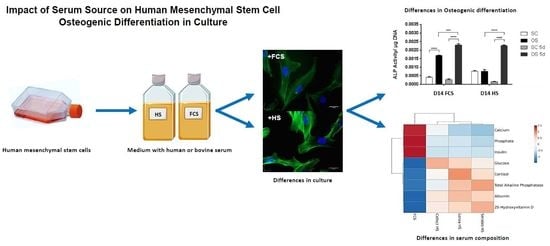
:1. Introduction
2. Results
2.1. MSC Culture in HS- or FCS -Based Medium
2.2. Transient Serum Exposure Regimes
2.3. Transient Serum Exposure Regimes in Primary MSC Cultures
3. Discussion
3.1. Effect of Serum Regimes on Cell Growth
3.2. Effect of Serum Regimes on Cell Differentiation
3.3. Transient Serum Approaches for Cell Differentiation
4. Materials and Methods
4.1. Cell Culture
4.2. Surface Marker Expression
4.3. Fluorescent Cell Staining
4.4. Apoptosis Assay
4.5. Cell Growth Measurements
4.6. Alizarin Red Staining and Quantification
4.7. Alkaline Phosphatase Assay
4.8. Serum Biochemical Analysis
4.9. RT-PCR
4.10. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| FCS | Fetal calf serum |
| HS | Human serum |
| MSC | Mesenchymal stem cell |
| PBS | Phosphate-buffered saline |
| SC | Standard culture |
Appendix A

References
- Lange, C.; Cakiroglu, F.; Spiess, A.N.; Cappallo-Obermann, H.; Dierlamm, J.; Zander, A.R. Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine. J. Cell. Physiol. 2007, 213, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Sottile, V.; Halleux, C.; Bassilana, F.; Keller, H.; Seuwen, K. Stem cell characteristics of human trabecular bone-derived cells. Bone 2002, 30, 699–704. [Google Scholar] [CrossRef]
- Bacigalupo, A. Management of acute graft-versus-host disease. Br. J. Haematol. 2007, 137, 87–98. [Google Scholar] [CrossRef]
- DiMarino, A.M.; Caplan, A.I.; Bonfield, T.L. Mesenchymal stem cells in tissue repair. Front. Immunol. 2013, 4, 201. [Google Scholar] [CrossRef]
- Kuznetsov, S.A.; Mankani, M.H.; Robey, P.G. Effect of serum on human bone marrow stromal cells: Ex vivo expansion and in vivo bone formation. Transplantation 2000, 70, 1780–1787. [Google Scholar] [CrossRef]
- Bieback, K.; Hecker, A.; Kocaoemer, A.; Lannert, H.; Schallmoser, K.; Strunk, D.; Klueter, H. Human Alternatives to Fetal Bovine Serum for the Expansion of Mesenchymal Stromal Cells from Bone Marrow. Stem Cells 2009, 27, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Wu, M.-L.; Hwang, S.-M. Optimization of serum free medium for cord blood mesenchymal stem cells. Biochem. Eng. J. 2007, 33, 1–9. [Google Scholar] [CrossRef]
- Meuleman, N.; Tondreau, T.; Delforge, A.; Dejeneffe, M.; Massy, M.; Libertalis, M.; Bron, D.; Lagneaux, L. Human marrow mesenchymal stem cell culture: Serum-free medium allows better expansion than classical alpha-MEM medium. Eur. J. Haematol. 2006, 76, 309–316. [Google Scholar] [CrossRef]
- Perez-Ilzarbe, M.; Diez-Campelo, M.; Aranda, P.; Tabera, S.; Lopez, T.; del Canizo, C.; Merino, J.; Moreno, C.; Andreu, E.J.; Prósper, F.; et al. Comparison of ex vivo expansion culture conditions of mesenchymal stem cells for human cell therapy. Transfusion 2009, 49, 1901–1910. [Google Scholar] [CrossRef] [PubMed]
- Aldahmash, A.; Haack-Sorensen, M.; Al-Nbaheen, M.; Harkness, L.; Abdallah, B.M.; Kassem, M. Human serum is as efficient as fetal bovine serum in supporting proliferation and differentiation of human multipotent stromal (mesenchymal) stem cells in vitro and in vivo. Stem Cell Rev. Rep. 2011, 7, 860–868. [Google Scholar] [CrossRef] [PubMed]
- France, L.; Scotchford, C.; Grant, D.; Rashidi, H.; Popov, A.; Sottile, V. Dual differentiation of human mesenchymal stem cell driven by transient serum exposure regimes. J. Tissue Eng. Regen. Med. 2014, 8, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Stute, N.; Holtz, K.; Bubenheim, M.; Lange, C.; Blake, F.; Zander, A.R. Autologous serum for isolation and expansion of human mesenchymal stem cells for clinical use. Exp. Hematol. 2004, 32. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Chung, J.-H.; Kwon, G.-Y.; Kim, K.-W.; Kim, S.; Chang, H. Effectiveness of autologous serum as an alternative to fetal bovine serum in adipose-derived stem cell engineering. Cell Tissue Bank 2013, 14, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Honmou, O.; Houkin, K.; Matsunaga, T.; Niitsu, Y.; Ishiai, S.; Onodera, R.; Waxman, S.G.; Kocsis, J.D. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain 2011, 134, 1790–1807. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, N.; Shiba, H.; Ozeki, Y.; Mouri, Y.; Niitani, M.; Inui, T.; Hayashi, H.; Suzuki, K.; Tanaka, S.; Kawaguchi, H.; et al. Human autologous serum obtained using a completely closed bag system as a substitute for foetal calf serum in human mesenchymal stem cell cultures. Cell Biol. Int. 2006, 30, 521–524. [Google Scholar] [CrossRef]
- Tateishi, K.; Ando, W.; Higuchi, C.; Hart, D.A.; Hashimoto, J.; Nakata, K.; Yoshikawa, H.; Nakamura, N. Comparison of human serum with fetal bovine serum for expansion and differentiation of human synovial MSC: Potential feasibility for clinical applications. Cell Transplant. 2008, 17, 549–557. [Google Scholar] [CrossRef]
- Abdallah, B.M.; Haack-Sorensen, M.; Fink, T.; Kassem, M. Inhibition of osteoblast differentiation but not adipocyte differentiation of mesenchymal stem cells by sera obtained from aged females. Bone 2006, 39, 181–188. [Google Scholar] [CrossRef]
- Chase, L.G.; Lakshmipathy, U.; Solchaga, L.A.; Rao, M.S.; Vemuri, M.C. A novel serum-free medium for the expansion of human mesenchymal stem cells. Stem Cell Res. Ther. 2010, 1, 8. [Google Scholar] [CrossRef]
- Tonti, G.A.; Mannello, F. From bone marrow to therapeutic applications: Different behaviour and genetic/epigenetic stability during mesenchymal stem cell expansion in autologous and foetal bovine sera? Int. J. Dev. Biol. 2008, 52, 1023–1032. [Google Scholar] [CrossRef]
- Pochampally, R.R.; Smith, J.R.; Ylostalo, J.; Prockop, D.J. Serum deprivation of human marrow stromal cells (hMSCs) selects for a subpopulation of early progenitor cells with enhanced expression of OCT-4 and other embryonic genes. Blood 2004, 103, 1647–1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pattappa, G.; Heywood, H.K.; De Bruijn, J.D.; Lee, D.A. The Metabolism of Human Mesenchymal Stem Cells During Proliferation and Differentiation. J. Cell. Physiol. 2011, 226, 2562–2570. [Google Scholar] [CrossRef] [PubMed]
- Roach, H.I. Association of matrix acid and alkaline phosphatases with mineralization of cartilage and endochondral bone. Histochem. J. 1999, 31, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Vater, C.; Kasten, P.; Stiehler, M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2011, 7, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef]
- Kyllonen, L.; Haimi, S.; Mannerstrom, B.; Huhtala, H.; Rajala, K.M.; Skottman, H.; Sándor, G.K.; Miettinen, S. Effects of different serum conditions on osteogenic differentiation of human adipose stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Merry, K.; Dodds, R.; Littlewood, A.; Gowen, M. Expression of osteopontin messenger-rna by osteoclasts and osteoblasts in modeling adult human bone. J. Cell. Sci. 1993, 104, 1013–1020. [Google Scholar] [PubMed]
- Girgis, C.M.; Baldock, P.A.; Downes, M. Vitamin D, muscle and bone: Integrating effects in development, aging and injury. Mol. Cell. Endocrinol. 2015, 410, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Goltzman, D.; Hendy, G.N.; White, J.H. Vitamin D and its receptor during late development. Biochim. Biophys. Acta 2015, 1849, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Staal, A.; Geertsma-Kleinekoort, W.M.C.; Van den Bemd, G.; Buurman, C.J.; Birkenhager, J.C.; Pols, H.A.P.; Van Leeuwen, J. Regulation of osteocalcin production and bone resorption by 1,25-dihydroxyvitamin D3 in mouse long bones: Interaction with the bone-derived growth factors TGF-β and IGF-I. J. Bone Miner. Res. 1998, 13, 36–43. [Google Scholar] [CrossRef]
- Misra, M.; Miller, K.K.; Almazan, C.; Ramaswamy, K.; Lapcharoensap, W.; Worley, M.; Neubauer, G.; Herzog, D.B.; Klibanski, A. Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J. Clin. Endocrinol. Metab. 2004, 89, 4972–4980. [Google Scholar] [CrossRef] [PubMed]
- Hauner, H.; Entenmann, G.; Wabitsch, M.; Gaillard, D.; Ailhaud, G.; Negrel, R.; Pfeiffer, E.F. Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J. Clin. Investig. 1989, 84, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Weil, B.R.; Abarbanell, A.M.; Herrmann, J.L.; Wang, Y.; Meldrum, D.R. High glucose concentration in cell culture medium does not acutely affect human mesenchymal stem cell growth factor production or proliferation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.; Heaney, R.P. Co-dependence of calcium and phosphorus for growth and bone development under conditions of varying deficiency. Bone 2003, 32, 532–540. [Google Scholar] [CrossRef]
- Coburn, S.P.; Mahuren, J.D.; Jain, M.; Zubovic, Y.; Wortsman, J. Alkaline phosphatase (EC 3.1.3.1) in serum is inhibited by physiological concentrations of inorganic phosphate. J. Clin. Endocrinol. Metab. 1998, 83, 3951–3957. [Google Scholar] [CrossRef] [PubMed]
- Sayers, A.; Lawlor, D.A.; Sattar, N.; Tobias, J.H. The association between insulin levels and cortical bone: Findings from a cross-sectional analysis of pQCT parameters in adolescents. J. Bone Miner. Res. 2012, 27, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Sendemir-Urkmez, A.; Jamison, R.D. The addition of biphasic calcium phosphate to porous chitosan scaffolds enhances bone tissue development in vitro. J. Biomed. Mater. Res. A 2007, 81, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Shahdadfar, A.; Fronsdal, K.; Haug, T.; Reinholt, F.P.; Brinchmann, J.E. In vitro expansion of human mesenchymal stem cells: Choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells 2005, 23, 1357–1366. [Google Scholar] [CrossRef]
- Kartsogiannis, V.; Ng, K.W. Cell lines and primary cell cultures in the study of bone cell biology. Mol. Cell. Endocrinol. 2004, 228, 79–102. [Google Scholar] [CrossRef]
- Sottile, V.; Thomson, A.; McWhir, J. In vitro osteogenic differentiation of human ES cells. Cloning Stem Cells 2003, 5, 149–155. [Google Scholar] [CrossRef]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Tataria, M.; Quarto, N.; Longaker, M.T.; Sylvester, K.G. Absence of the p53 tumor suppressor gene promotes osteogenesis in mesenchymal stem cells. J. Pediatr. Surg. 2006, 41, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.H.; Jiang, J.J.; Sottile, V.; McWhir, J.; Lebkowski, J.; Carpenter, M.K. Immortalized fibroblast-like cells derived from human embryonic stem cells support undifferentiated cell growth. Stem Cells 2004, 22, 972–980. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, A.; Scotchford, C.; Grant, D.; Sottile, V. Impact of Serum Source on Human Mesenchymal Stem Cell Osteogenic Differentiation in Culture. Int. J. Mol. Sci. 2019, 20, 5051. https://doi.org/10.3390/ijms20205051
Popov A, Scotchford C, Grant D, Sottile V. Impact of Serum Source on Human Mesenchymal Stem Cell Osteogenic Differentiation in Culture. International Journal of Molecular Sciences. 2019; 20(20):5051. https://doi.org/10.3390/ijms20205051
Chicago/Turabian StylePopov, Alexander, Colin Scotchford, David Grant, and Virginie Sottile. 2019. "Impact of Serum Source on Human Mesenchymal Stem Cell Osteogenic Differentiation in Culture" International Journal of Molecular Sciences 20, no. 20: 5051. https://doi.org/10.3390/ijms20205051