Shielding of Hepatitis B Virus-Like Nanoparticle with Poly(2-Ethyl-2-Oxazoline)
Abstract
:1. Introduction
2. Results
2.1. Synthesis of PEtOx-NH2
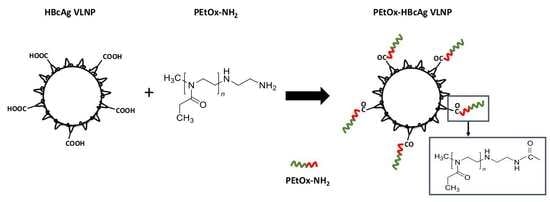
2.2. Conjugation of PEtOx-NH2 to HBcAg VLNPs
2.3. Dynamic Light Scattering (DLS) and Zeta Potential of HBcAg VLNPs
2.4. Colloidal Stability of HBcAg and PEtOx-HBcAg VLNPs
2.5. Antigenicity of PEtOx-Conjugated HBcAg VLNPs
2.6. Comparison of the Antigenicity of PEtOx-Conjugated HBcAg VLNPs and PEGylated HBcAg VLNPs
3. Discussion
4. Materials and Methods
4.1. Expression and Purification of HBcAg VLNPs
4.2. Synthesis of PEtOx-NH2
4.3. Characterization of PEtOx-NH2 with NMR and Mass Spectrometry
4.4. Conjugation of PEtOx-NH2 to HBcAg VLNPs
4.5. UV-Visible Spectroscopy
4.6. Dynamic Light Scattering (DLS) and Zeta Potential Measurement
4.7. Colloidal Stability of HBcAg and PEtOx-HBcAg VLNPs
4.8. Antigenicity of PEtOx-HBcAg VLNPs
4.9. Comparison of the Antigenicity of PEtOx-Conjugated HBcAg VLNPs and PEGylated HBcAg VLNPs
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rohovie, M.J.; Nagasawa, M.; Swartz, J.R. Virus-like particles: Next-generation nanoparticles for targeted therapeutic delivery. Bioeng. Transl. Med. 2017, 2, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, J.; Tullman-Ercek, D. Production and applications of engineered viral capsids. Appl. Microbiol. Biotechnol. 2014, 98, 5847–5858. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Tey, B.T.; Ho, K.L.; Tejo, B.A.; Tan, W.S. Nanoglue: An alternative way to display cell-internalizing peptide at the spikes of hepatitis B virus core nanoparticles for cell-targeting delivery. Mol. Pharm. 2012, 9, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Biabanikhankahdani, R.; Alitheen, N.B.M.; Ho, K.L.; Tan, W.S. pH-responsive virus-like nanoparticles with enhanced tumour-targeting ligands for cancer drug delivery. Sci. Rep. 2016, 6, 37891. [Google Scholar] [CrossRef]
- Biabanikhankahdani, R.; Bayat, S.; Ho, K.L.; Alitheen, N.B.M.; Tan, W.S. A simple add-and-display method for immobilisation of cancer drug on His-tagged virus-like nanoparticles for controlled drug delivery. Sci. Rep. 2017, 7, 5303. [Google Scholar] [CrossRef]
- Biabanikhankahdani, R.; Ho, K.L.; Alitheen, N.B.M.; Tan, W.S. A dual bioconjugated virus-like nanoparticle as a drug delivery system and comparison with a pH-responsive delivery system. Nanomaterials 2018, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Ilinskaya, A.N.; Dobrovolskaia, M.A. Understanding the immunogenicity and antigenicity of nanomaterials: Past, present and future. Toxicol. Appl. Pharmacol. 2016, 299, 70–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowther, R.A.; Kiselev, N.A.; Böttcher, B.; Berriman, J.A.; Borisova, G.P.; Ose, V.; Pumpens, P. Three-dimensional structure of hepatitis B virus core particles determined by electron cryomicroscopy. Cell 1994, 77, 943–950. [Google Scholar] [CrossRef]
- Lee, K.W.; Tan, W.S. Recombinant hepatitis B virus core particles: Association, dissociation and encapsidation of green fluorescent protein. J. Virol. Methods 2008, 151, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Dhason, M.S.; Wang, J.C.-Y.; Hagan, M.F.; Zlotnick, A. Differential assembly of hepatitis B virus core protein on single- and double-stranded nucleic acid suggest the dsDNA-filled core is spring-loaded. Virology 2012, 430, 20–29. [Google Scholar] [CrossRef]
- Lee, K.W.; Tey, B.T.; Ho, K.L.; Tan, W.S. Delivery of chimeric hepatitis B core particles into liver cells. J. Appl. Microbiol. 2012, 112, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Strods, A.; Ose, V.; Bogans, J.; Cielens, I.; Kalnins, G.; Radovica, I.; Kazaks, A.; Pumpens, P.; Renhofa, R. Preparation by alkaline treatment and detailed characterisation of empty hepatitis B virus core particles for vaccine and gene therapy applications. Sci. Rep. 2015, 5, 11639. [Google Scholar] [CrossRef] [PubMed]
- Gan, B.K.; Yong, C.Y.; Ho, K.L.; Omar, A.R.; Alitheen, N.B.; Tan, W.S. Targeted delivery of cell penetrating peptide virus-like nanoparticles to skin cancer cells. Sci. Rep. 2018, 8, 8499. [Google Scholar] [CrossRef] [PubMed]
- Milich, D.; McLachlan, A. The nucleocapsid of hepatitis B virus is both a T-cell-independent and a T-cell-dependent antigen. Science 1986, 234, 1398–1401. [Google Scholar] [CrossRef] [PubMed]
- Milich, D.R.; Chen, M.; Schodel, F.; Peterson, D.L.; Jones, J.E.; Hughes, J.L. Role of B cells in antigen presentation of the hepatitis B core. Proc. Natl. Acad. Sci. USA 1997, 94, 14648–14653. [Google Scholar] [CrossRef] [Green Version]
- Belnap, D.M.; Watts, N.R.; Conway, J.F.; Cheng, N.; Stahl, S.J.; Wingfield, P.T.; Steven, A.C. Diversity of core antigen epitopes of hepatitis B virus. Proc. Natl. Acad. Sci. USA 2003, 100, 10884–10889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gref, R.; Minamitake, Y.; Peracchia, M.; Trubetskoy, V.; Torchilin, V.; Langer, R. Biodegradable long-circulating polymeric nanospheres. Science 1994, 263, 1600–1603. [Google Scholar] [CrossRef]
- Illum, L.; Davis, S.S. The organ uptake of intravenously administered colloidal particles can be altered using a non-ionic surfactant (Poloxamer 338). FEBS Lett. 1984, 167, 79–82. [Google Scholar] [CrossRef] [Green Version]
- Kaul, G.; Amiji, M. Long-circulating poly(ethylene glycol)-modified gelatin nanoparticles for intracellular delivery. Pharm. Res. 2002, 19, 1061–1067. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; McNeil, S.E. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007, 2, 469–478. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Aggarwal, P.; Hall, J.B.; McNeil, S.E. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol. Pharm. 2008, 5, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molineux, G. Pegylation: Engineering improved pharmaceuticals for enhanced therapy. Cancer Treat. Rev. 2002, 28, 13–16. [Google Scholar] [CrossRef]
- Walkey, C.D.; Chan, W.C.W. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, B.; del Pino, P.; Maffre, P.; Hartmann, R.; Gallego, M.; Rivera-Fernández, S.; de la Fuente, J.M.; Nienhaus, G.U.; Parak, W.J. Surface functionalization of nanoparticles with polyethylene glycol: Effects on protein adsorption and cellular uptake. ACS Nano 2015, 9, 6996–7008. [Google Scholar] [CrossRef] [PubMed]
- Dams, E.T.M.; Laverman, P.; Oyen, W.J.G.; Storm, G.; Scherphof, G.L.; van der Meer, J.W.M.; Corstens, F.H.M.; Boerman, O.C. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J. Pharmacol. Exp. Ther. 2000, 292, 1071–1079. [Google Scholar] [PubMed]
- Laverman, P.; Carstens, M.G.; Boerman, O.C.; Dams, E.T.M.; Oyen, W.J.G.; van Rooijen, N.; Corstens, F.H.M.; Storm, G. Factors affecting the accelerated blood clearance of polyethylene glycol-liposomes upon repeated injection. J. Pharmacol. Exp. Ther. 2001, 298, 607–612. [Google Scholar]
- Ishida, T.; Maeda, R.; Ichihara, M.; Mukai, Y.; Motoki, Y.; Manabe, Y.; Irimura, K.; Kiwada, H. The accelerated clearance on repeated injection of pegylated liposomes in rats: Laboratory and histopathological study. Cell. Mol. Biol. Lett. 2002, 7, 286. [Google Scholar]
- Nagao, A.; Abu Lila, A.S.; Ishida, T.; Kiwada, H. Abrogation of the accelerated blood clearance phenomenon by SOXL regimen: Promise for clinical application. Int. J. Pharm. 2013, 441, 395–401. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Wang, L.; Yang, Q.; Tang, W.; She, Z.; Deng, Y. A frustrating problem: Accelerated blood clearance of PEGylated solid lipid nanoparticles following subcutaneous injection in rats. Eur. J. Pharm. Biopharm. 2012, 81, 506–513. [Google Scholar] [CrossRef]
- Suzuki, T.; Ichihara, M.; Hyodo, K.; Yamamoto, E.; Ishida, T.; Kiwada, H.; Ishihara, H.; Kikuchi, H. Accelerated blood clearance of PEGylated liposomes containing doxorubicin upon repeated administration to dogs. Int. J. Pharm. 2012, 436, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, Q.; Wang, L.; Zhou, X.; Zhao, Y.; Deng, Y. Repeated injections of PEGylated liposomal topotecan induces accelerated blood clearance phenomenon in rats. Eur. J. Pharm. Sci. 2012, 45, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Ichihara, M.; Yoshioka, Y.; Ishida, T.; Nakagawa, S.; Kiwada, H. Intravenous administration of polyethylene glycol-coated (PEGylated) proteins and PEGylated adenovirus elicits an anti-PEG immunoglobulin M response. Biol. Pharm. Bull. 2012, 35, 1336–1342. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Shimizu, T.; Mima, Y.; Abu Lila, A.S.; Ishida, T.; Kiwada, H. Generation, characterization and in vivo biological activity of two distinct monoclonal anti-PEG IgMs. Toxicol. Appl. Pharmacol. 2014, 277, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboom, R. 50 years of poly(2-oxazoline)s. Eur. Polym. J. 2017, 88, 448–450. [Google Scholar] [CrossRef]
- Chapman, R.G.; Ostuni, E.; Takayama, S.; Holmlin, R.E.; Yan, L.; Whitesides, G.M. Surveying for surfaces that resist the adsorption of proteins. J. Am. Chem. Soc. 2000, 122, 8303–8304. [Google Scholar] [CrossRef]
- Abu Lila, A.S.; Kiwada, H.; Ishida, T. The accelerated blood clearance (ABC) phenomenon: Clinical challenge and approaches to manage. J. Control. Release 2013, 172, 38–47. [Google Scholar] [CrossRef]
- Verhoef, J.J.F.; Carpenter, J.F.; Anchordoquy, T.J.; Schellekens, H. Potential induction of anti-PEG antibodies and complement activation toward PEGylated therapeutics. Drug Discov. Today 2014, 19, 1945–1952. [Google Scholar] [CrossRef]
- Luxenhofer, R.; Han, Y.; Schulz, A.; Tong, J.; He, Z.; Kabanov, A.V.; Jordan, R. Poly(2-oxazoline)s as polymer therapeutics. Macromol. Rapid Commun. 2012, 33, 1613–1631. [Google Scholar] [CrossRef]
- de la Rosa, V.R. Poly(2-oxazoline)s as materials for biomedical applications. J. Mater. Sci. Mater. Med. 2014, 25, 1211–1225. [Google Scholar] [CrossRef]
- Bludau, H.; Czapar, A.E.; Pitek, A.S.; Shukla, S.; Jordan, R.; Steinmetz, N.F. POxylation as an alternative stealth coating for biomedical applications. Eur. Polym. J. 2017, 88, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Moreadith, R.W.; Viegas, T.X.; Bentley, M.D.; Harris, J.M.; Fang, Z.; Yoon, K.; Dizman, B.; Weimer, R.; Rae, B.P.; Li, X.; et al. Clinical development of a poly(2-oxazoline) (POZ) polymer therapeutic for the treatment of Parkinson’s disease—Proof of concept of POZ as a versatile polymer platform for drug development in multiple therapeutic indications. Eur. Polym. J. 2017, 88, 524–552. [Google Scholar] [CrossRef]
- Viegas, T.X.; Bentley, M.D.; Harris, J.M.; Fang, Z.; Yoon, K.; Dizman, B.; Weimer, R.; Mero, A.; Pasut, G.; Veronese, F.M. Polyoxazoline: Chemistry, properties, and applications in drug delivery. Bioconjug. Chem. 2011, 22, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Luxenhofer, R.; López-García, M.; Frank, A.; Kessler, H.; Jordan, R. First poly(2-oxazoline)-peptide conjugate for targeted radionuclide cancer therapy. PMSE Prepr. 2006, 95, 283–284. [Google Scholar]
- Mero, A.; Fang, Z.; Pasut, G.; Veronese, F.M.; Viegas, T.X. Selective conjugation of poly(2-ethyl 2-oxazoline) to granulocyte colony stimulating factor. J. Control. Release 2012, 159, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Moreadith, R.W.; Viegas, T.X.; Standaert, D.G.; Bentley, M.D.; Fang, Z.; Dizman, B.; Yoon, K.; Weimer, R.; Harris, J.M.; Ravenscroft, P. SER-214, a novel polymer-conjugated rotigotine formulation affords greatly extended duration of anti-parkinsonian effect and enhanced plasma exposure following a single administration in rodents and primates. In Proceedings of the 16th international conference of Parkinson’s disease and movement disorders, movement disorder society, Dublin, Ireland, 17–21 June 2012; pp. 17–21. [Google Scholar]
- Sedlacek, O.; Monnery, B.D.; Filippov, S.K.; Hoogenboom, R.; Hruby, M. Poly(2-Oxazoline)s - Are they more advantageous for biomedical applications than other polymers? Macromol. Rapid Commun. 2012, 33, 1648–1662. [Google Scholar] [CrossRef] [PubMed]
- Karadag, K.; Yamada, S.; Endo, T. Synthesis of poly(2-ethyl-2-oxazoline)-block-polypeptide copolymers by combination of ring-opening polymerization of oxazoline and polycondensation of activated urethane derivatives of α-amino acids. Polym. Bull. 2018, 75, 5075–5088. [Google Scholar] [CrossRef]
- Carrstensen, H.; Müller, R.H.; Müller, B.W. Particle size, surface hydrophobicity and interaction with serum of parenteral fat emulsions and model drug carriers as parameters related to RES uptake. Clin. Nutr. 1992, 11, 289–297. [Google Scholar] [CrossRef]
- Müller, R.H.; Wallis, K.H.; Tröster, S.D.; Kreuter, J. In vitro characterization of poly(methyl-methaerylate) nanoparticles and correlation to their in vivo fate. J. Control. Release 1992, 20, 237–246. [Google Scholar] [CrossRef]
- Norman, M.E.; Williams, P.; Illum, L. Human serum albumin as a probe for surface conditioning (opsonization) of block copolymer-coated microspheres. Biomaterials 1992, 13, 841–849. [Google Scholar] [CrossRef]
- Owens, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Putz, M.V.; Duda-Seiman, C.; Duda-Seiman, D.; Putz, A.-M.; Alexandrescu, I.; Mernea, M.; Avram, S. Chemical structure-biological activity models for pharmacophores’ 3D-interactions. Int. J. Mol. Sci. 2016, 17, 1087. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.S.; Dyson, M.R.; Murray, K. Hepatitis B virus core antigen: Enhancement of its production in Escherichia coli, and interaction of the core particles with the viral surface antigen. Biol. Chem. 2003, 384, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Dyson, M.R.; Murray, K. Selection of peptide inhibitors of interactions involved in complex protein assemblies: Association of the core and surface antigens of hepatitis B virus. Proc. Natl. Acad. Sci. USA 1995, 92, 2194–2198. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Harmuth, S.; Barth, E.R.; Wurm, E.; Fobbe, R.; Sickmann, A.; Krumm, C.; Tiller, J.C. Conjugation of ciprofloxacin with poly(2-oxazoline)s and polyethylene glycol via end groups. Bioconjug. Chem. 2015, 26, 1950–1962. [Google Scholar] [CrossRef] [PubMed]








| Sample | Z-Average Diameter (nm) | Zeta Potential (mV) |
|---|---|---|
| HBcAg | 34.47 ± 0.23 | −38.40 ± 2.76 |
| PEtOx-HBcAg | 35.55 ± 1.95 | −31.00 ± 1.71 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fam, S.Y.; Chee, C.F.; Yong, C.Y.; Ho, K.L.; Mariatulqabtiah, A.R.; Lau, H.Y.; Tan, W.S. Shielding of Hepatitis B Virus-Like Nanoparticle with Poly(2-Ethyl-2-Oxazoline). Int. J. Mol. Sci. 2019, 20, 4903. https://doi.org/10.3390/ijms20194903
Fam SY, Chee CF, Yong CY, Ho KL, Mariatulqabtiah AR, Lau HY, Tan WS. Shielding of Hepatitis B Virus-Like Nanoparticle with Poly(2-Ethyl-2-Oxazoline). International Journal of Molecular Sciences. 2019; 20(19):4903. https://doi.org/10.3390/ijms20194903
Chicago/Turabian StyleFam, See Yee, Chin Fei Chee, Chean Yeah Yong, Kok Lian Ho, Abdul Razak Mariatulqabtiah, Han Yih Lau, and Wen Siang Tan. 2019. "Shielding of Hepatitis B Virus-Like Nanoparticle with Poly(2-Ethyl-2-Oxazoline)" International Journal of Molecular Sciences 20, no. 19: 4903. https://doi.org/10.3390/ijms20194903